
Which of the following is a polar molecule?
A. \[Xe{F_4}\]
B. \[\;B{F_3}\]
C. \[\;S{F_4}\]
D. \[\;Si{F_4}\]
Answer
589.2k+ views
Hint: To be a polar molecule, a permanent dipole moment should be present. The dipole moment is a quantity to measure the polarity of the molecule. The dipole moment of a molecule depends upon the geometry of the molecule.
Complete step by step answer:
To predict the structure of a molecule the hybridization of the molecule should be known. The formula to calculate the hybridization of the central atom of the molecule is, \[H = \dfrac{1}{2}\left[ {V + X - C + A} \right]\] . where V is the number of valence electrons of the central atom, X is the number of monovalent atoms attached to the central atom, C is the total cationic charge and A is the total anionic charge, H is the hybridization.
Now, for \[B{F_3}\] the hybridization is,
\[
H = \dfrac{1}{2}\left[ {V + X - C + A} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ {3 + 3 - 0 + 0} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ {3 + 3} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ 6 \right] \\
\Rightarrow H = 3 \\
\]
For \[H = 3\] , the hybridization would be \[s{p^2}\] , so the geometry of \[B{F_3}\] is trigonal planar. here the central atom boron has 3 bond pairs and 0 lone pair. Here all bond dipoles cancel out to each other and the overall dipole moment becomes zero.
Now, for \[Si{F_4}\] the hybridization is,
\[
H = \dfrac{1}{2}\left[ {V + X - C + A} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ {4 + 4 - 0 + 0} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ 8 \right] \\
\Rightarrow H = 4 \\
\]
For \[H = 4\] , the hybridization would be \[s{p^3}\] , so the geometry of \[Si{F_4}\] is tetrahedral. In this case, the central atom silicon has 4 bond pairs and 0 lone pair, therefore, the structure should be tetrahedral and as it is a regular tetrahedral overall dipole moment is zero.
Now, for \[Xe{F_4}\] the hybridization is,
\[
H = \dfrac{1}{2}\left[ {V + X - C + A} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ {8 + 4 - 0 + 0} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ {12} \right] \\
\Rightarrow H = 6 \\
\]
For \[H = 6\] , the hybridization would be \[s{p^3}{d^2}\] , so the geometry of \[Xe{F_4}\] is tetrahedral. In this case, the central atom chlorine has 4 bond pairs and 2 lone pairs, therefore, due to this structure the overall dipole moment is zero.
For, \[\;S{F_4}\] the hybridization is,
\[
H = \dfrac{1}{2}\left[ {V + X - C + A} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ {6 + 4 - 0 + 0} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ {10} \right] \\
\Rightarrow H = 5 \\
\]
For \[H = 5\] , the hybridization would be \[s{p^3}d\] , so the geometry of \[\;S{F_4}\] is Trigonal bipyramidal. In this case, the central atom chlorine has 4 bond pairs and 1 lone pair, therefore, the shape is a see-saw. Due to this shape, it has a permanent dipole moment. The structure is shown below,
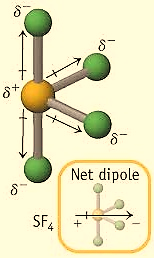
So, the correct answer is C.
Note:Remember that when a lone pair is present, then the molecular geometry will be the three-dimensional arrangement of the atoms without the lone pair. If there is no lone pair only bond pairs then the number of bond pairs can be equal to the hybridization number.
Complete step by step answer:
To predict the structure of a molecule the hybridization of the molecule should be known. The formula to calculate the hybridization of the central atom of the molecule is, \[H = \dfrac{1}{2}\left[ {V + X - C + A} \right]\] . where V is the number of valence electrons of the central atom, X is the number of monovalent atoms attached to the central atom, C is the total cationic charge and A is the total anionic charge, H is the hybridization.
| Hybridization number(H) | Hybridization | geometry |
| 2 | Sp | linear |
| 3 | \[s{p^2}\] | Trigonal planar |
| 4 | \[s{p^3}\] | Tetrahedral |
| 5 | \[s{p^3}d\] | Trigonal bipyramidal |
| 6 | \[s{p^3}{d^2}\] | Octahedral |
| 7 | \[s{p^3}{d^3}\] | Pentagonal bipyramidal |
Now, for \[B{F_3}\] the hybridization is,
\[
H = \dfrac{1}{2}\left[ {V + X - C + A} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ {3 + 3 - 0 + 0} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ {3 + 3} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ 6 \right] \\
\Rightarrow H = 3 \\
\]
For \[H = 3\] , the hybridization would be \[s{p^2}\] , so the geometry of \[B{F_3}\] is trigonal planar. here the central atom boron has 3 bond pairs and 0 lone pair. Here all bond dipoles cancel out to each other and the overall dipole moment becomes zero.
Now, for \[Si{F_4}\] the hybridization is,
\[
H = \dfrac{1}{2}\left[ {V + X - C + A} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ {4 + 4 - 0 + 0} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ 8 \right] \\
\Rightarrow H = 4 \\
\]
For \[H = 4\] , the hybridization would be \[s{p^3}\] , so the geometry of \[Si{F_4}\] is tetrahedral. In this case, the central atom silicon has 4 bond pairs and 0 lone pair, therefore, the structure should be tetrahedral and as it is a regular tetrahedral overall dipole moment is zero.
Now, for \[Xe{F_4}\] the hybridization is,
\[
H = \dfrac{1}{2}\left[ {V + X - C + A} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ {8 + 4 - 0 + 0} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ {12} \right] \\
\Rightarrow H = 6 \\
\]
For \[H = 6\] , the hybridization would be \[s{p^3}{d^2}\] , so the geometry of \[Xe{F_4}\] is tetrahedral. In this case, the central atom chlorine has 4 bond pairs and 2 lone pairs, therefore, due to this structure the overall dipole moment is zero.
For, \[\;S{F_4}\] the hybridization is,
\[
H = \dfrac{1}{2}\left[ {V + X - C + A} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ {6 + 4 - 0 + 0} \right] \\
\Rightarrow H = \dfrac{1}{2}\left[ {10} \right] \\
\Rightarrow H = 5 \\
\]
For \[H = 5\] , the hybridization would be \[s{p^3}d\] , so the geometry of \[\;S{F_4}\] is Trigonal bipyramidal. In this case, the central atom chlorine has 4 bond pairs and 1 lone pair, therefore, the shape is a see-saw. Due to this shape, it has a permanent dipole moment. The structure is shown below,

So, the correct answer is C.
Note:Remember that when a lone pair is present, then the molecular geometry will be the three-dimensional arrangement of the atoms without the lone pair. If there is no lone pair only bond pairs then the number of bond pairs can be equal to the hybridization number.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




