
Which of the following is a non-aromatic compound?
A.
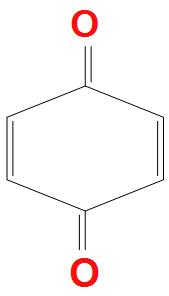
B.
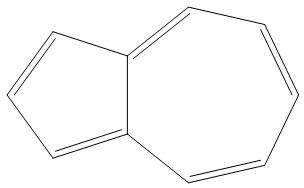
C.
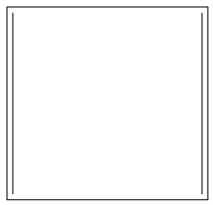
D.
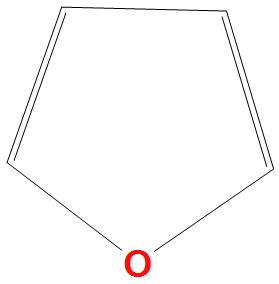




Answer
567.3k+ views
Hint: Aromatic compounds are those that obeys Huckel’s rule, are planar and will show cyclic delocalisation. Huckel’s rule is $\left( 4n+2 \right)\pi {{e}^{-}}$, where n is the number of benzene rings. For n=1, there should be 6 pi electrons. For n=2, there should be 10 pi electrons and so on.
Complete Solution :
- A.
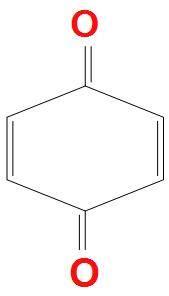
There are carbonyl pi electrons present, but these do not resonate within the ring, that’s why they are not aromatic. Second reason is that there is also no proper conjugation present, and only cross conjugation is present. It doesn’t obey Huckel’s rule for aromaticity. Hence, it is a non-aromatic compound.
- B.
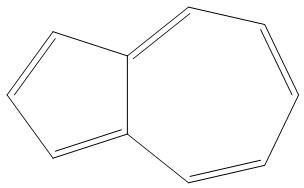
It is having 10 pi electron, and is an aromatic compound as it obeys Huckel’s rule$\left( 4n+2 \right)\pi {{e}^{-}}$:
\[\begin{align}
& \left( 4\times 2+2 \right)\pi {{e}^{-}} \\
& =10\pi {{e}^{-}} \\
\end{align}\]
- Here, we can see that as n= 2, that two rings are present and there are 10pi electrons, hence it is an aromatic compound.
\[\begin{align}
& \left( 4\times 2+2 \right)\pi {{e}^{-}} \\
& =10\pi {{e}^{-}} \\
\end{align}\]
- C.
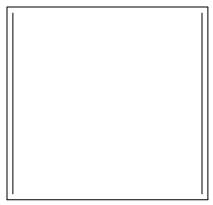
In this we can see that, there are two pi bonds, so there are four pi electrons. Hence, it will not obey Huckel’s rule $\left( 4n+2 \right)\pi {{e}^{-}}$:
$\begin{align}
& \left( 4\times 1+2 \right)\pi e- \\
& =6\pi {{e}^{-}} \\
\end{align}$
As there are 4 pi electrons, it will not be aromatic.
- It is anti- aromatic compound. Anti-aromatic are not non-aromatic compounds. They are also planar like aromatic compounds and also have conjugated double bonds but the number of pi electrons are 4n not 4n+2 which is an essential criteria for aromatic compounds according to the Huckel’s rule. So they are called anti-aromatic compounds.
- D.
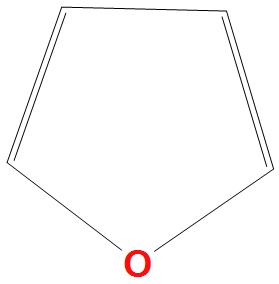
Here a lone pair of electrons are present on oxygen, which will delocalise the benzene ring, hence there are six pi electrons present that are two pi bonds and one lone pair of electrons.
That’s why it will obey Huckel’s rule $\left( 4n+2 \right)\pi {{e}^{-}}$:
$\begin{align}
& \left( 4\times 1+2 \right)\pi e- \\
& =6\pi {{e}^{-}} \\
\end{align}$
We can say that it is aromatic compound as it obeys Huckel’s rule
So, the correct answer is “Option A”.
Note: - We can say that an aromatic compound is one which obeys the criteria’s like:
- It should have the capability to undergo substitution instead of addition reactions.
- It must follow Huckel’s rule that is
- It must be cyclic, planar structure
- It must have stability due to the pi electron delocalization.
Complete Solution :
- A.

There are carbonyl pi electrons present, but these do not resonate within the ring, that’s why they are not aromatic. Second reason is that there is also no proper conjugation present, and only cross conjugation is present. It doesn’t obey Huckel’s rule for aromaticity. Hence, it is a non-aromatic compound.
- B.

It is having 10 pi electron, and is an aromatic compound as it obeys Huckel’s rule$\left( 4n+2 \right)\pi {{e}^{-}}$:
\[\begin{align}
& \left( 4\times 2+2 \right)\pi {{e}^{-}} \\
& =10\pi {{e}^{-}} \\
\end{align}\]
- Here, we can see that as n= 2, that two rings are present and there are 10pi electrons, hence it is an aromatic compound.
\[\begin{align}
& \left( 4\times 2+2 \right)\pi {{e}^{-}} \\
& =10\pi {{e}^{-}} \\
\end{align}\]
- C.

In this we can see that, there are two pi bonds, so there are four pi electrons. Hence, it will not obey Huckel’s rule $\left( 4n+2 \right)\pi {{e}^{-}}$:
$\begin{align}
& \left( 4\times 1+2 \right)\pi e- \\
& =6\pi {{e}^{-}} \\
\end{align}$
As there are 4 pi electrons, it will not be aromatic.
- It is anti- aromatic compound. Anti-aromatic are not non-aromatic compounds. They are also planar like aromatic compounds and also have conjugated double bonds but the number of pi electrons are 4n not 4n+2 which is an essential criteria for aromatic compounds according to the Huckel’s rule. So they are called anti-aromatic compounds.
- D.

Here a lone pair of electrons are present on oxygen, which will delocalise the benzene ring, hence there are six pi electrons present that are two pi bonds and one lone pair of electrons.
That’s why it will obey Huckel’s rule $\left( 4n+2 \right)\pi {{e}^{-}}$:
$\begin{align}
& \left( 4\times 1+2 \right)\pi e- \\
& =6\pi {{e}^{-}} \\
\end{align}$
We can say that it is aromatic compound as it obeys Huckel’s rule
So, the correct answer is “Option A”.
Note: - We can say that an aromatic compound is one which obeys the criteria’s like:
- It should have the capability to undergo substitution instead of addition reactions.
- It must follow Huckel’s rule that is
- It must be cyclic, planar structure
- It must have stability due to the pi electron delocalization.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




