
Which of the following is a diatomic gas?
(A) Hydrogen
(B) Oxygen
(C) Chlorine
(D) All of the above
Answer
538.2k+ views
Hint: In a free state and at standard temperature and pressure, some elements exist as diatomic molecules to achieve stability. In these diatomic molecules, both the atoms are of the same element and hence are homonuclear.
Complete answer:
To determine whether the gas exists as a diatomic molecule, we need to check the stability of the diatomic molecule formed.
(A) Hydrogen atom has 1 electron in its valence shell and needs 1 more electron to form a complete outermost shell. So when two hydrogen atoms form a bond, both the hydrogen atoms share two electrons between them, forming a covalent single bond and completing the octet.
Its Lewis structure is as follows:

Hence ${{H}_{2}}$ is stable and exists as a diatomic gas.
(B) Oxygen atom has 6 electrons in its valence shell and needs 2 more electrons to form a complete outermost octet shell. So, when 2 oxygen atoms form a bond, both the oxygen atoms share 4 electrons between them, forming a covalent double bond and completing the octet.
Its Lewis structure is as follows:
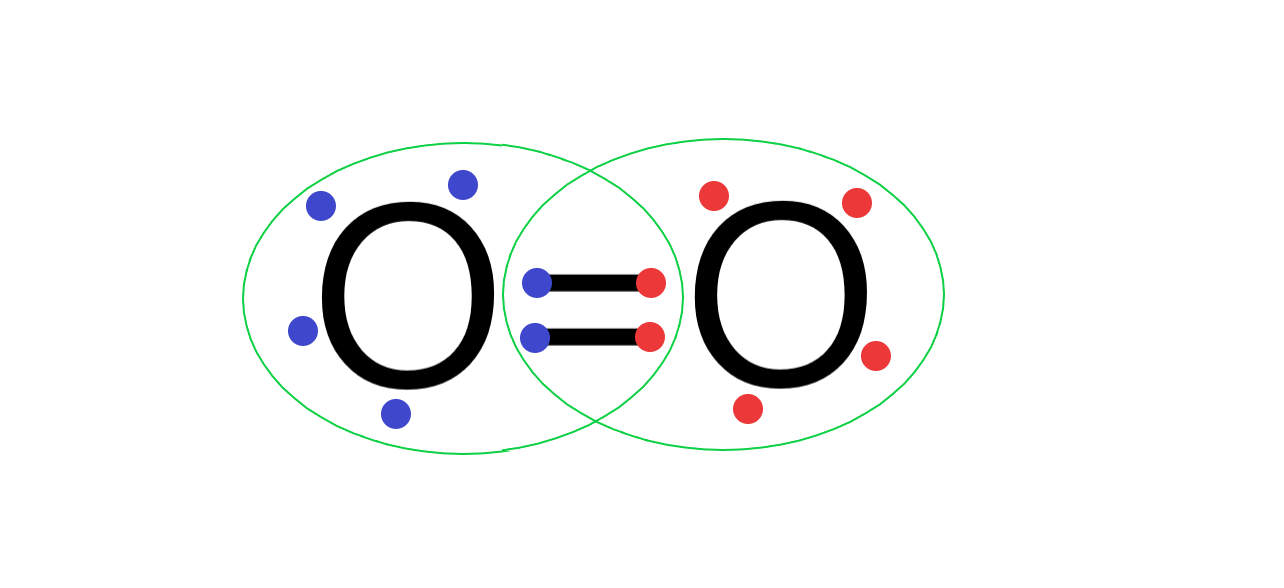
Hence ${{O}_{2}}$ is stable and exists as a diatomic gas.
(C) Chlorine atom has 7 electrons in its valence shell and needs 1 more electron to form a complete outermost octet shell. So when 2 chlorine atoms form a bond, both the chlorine atoms share 2 electrons between them, forming a covalent single bond and completing the octet.
Its Lewis structure is as follows:

Hence $C{{l}_{2}}$ is stable and exists as a diatomic gas.
So, the correct answer is option (D) All of the above.
Note:
It should be noted that other than chlorine $C{{l}_{2}}$, oxygen ${{O}_{2}}$, and hydrogen ${{H}_{2}}$, the only other gases that exist as homonuclear diatomic molecules at STP are fluorine ${{F}_{2}}$ and nitrogen ${{N}_{2}}$.
At temperatures higher than STP, bromine $B{{r}_{2}}$ , and iodine ${{I}_{2}}$ also exist as diatomic molecules.
Complete answer:
To determine whether the gas exists as a diatomic molecule, we need to check the stability of the diatomic molecule formed.
(A) Hydrogen atom has 1 electron in its valence shell and needs 1 more electron to form a complete outermost shell. So when two hydrogen atoms form a bond, both the hydrogen atoms share two electrons between them, forming a covalent single bond and completing the octet.
Its Lewis structure is as follows:

Hence ${{H}_{2}}$ is stable and exists as a diatomic gas.
(B) Oxygen atom has 6 electrons in its valence shell and needs 2 more electrons to form a complete outermost octet shell. So, when 2 oxygen atoms form a bond, both the oxygen atoms share 4 electrons between them, forming a covalent double bond and completing the octet.
Its Lewis structure is as follows:

Hence ${{O}_{2}}$ is stable and exists as a diatomic gas.
(C) Chlorine atom has 7 electrons in its valence shell and needs 1 more electron to form a complete outermost octet shell. So when 2 chlorine atoms form a bond, both the chlorine atoms share 2 electrons between them, forming a covalent single bond and completing the octet.
Its Lewis structure is as follows:

Hence $C{{l}_{2}}$ is stable and exists as a diatomic gas.
So, the correct answer is option (D) All of the above.
Note:
It should be noted that other than chlorine $C{{l}_{2}}$, oxygen ${{O}_{2}}$, and hydrogen ${{H}_{2}}$, the only other gases that exist as homonuclear diatomic molecules at STP are fluorine ${{F}_{2}}$ and nitrogen ${{N}_{2}}$.
At temperatures higher than STP, bromine $B{{r}_{2}}$ , and iodine ${{I}_{2}}$ also exist as diatomic molecules.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




