
Which of the following is 2,4 –dimethylhexane?
A. $C{{H}_{3}}-C{{(C{{H}_{3}})}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}$
B. $C{{H}_{3}}-CH(C{{H}_{3}})-CH(C{{H}_{3}})-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}$
C. $C{{H}_{3}}-CH(C{{H}_{3}})-C{{H}_{2}}-CH(C{{H}_{3}})-C{{H}_{2}}-C{{H}_{3}}$
D. $C{{H}_{3}}-CH(C{{H}_{3}})-C{{H}_{2}}-C{{H}_{2}}-CH(C{{H}_{3}})-C{{H}_{3}}$
Answer
560.7k+ views
Hint: According to IUPAC (International Union of Pure and Applied Chemistry), whenever we are going to write the IUPAC name of a compound, we have to give numbering first to functional groups or highly substituted carbon. Means lower numbering should be a functional group or highly substituted carbon present in the molecule or compound.
Complete step by step answer:
- In the question it is given to identify the structure of the compound with the IUPAC name 2,4 –dimethylhexane among the given options.
- Coming to given options, option A, $C{{H}_{3}}-C{{(C{{H}_{3}})}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}$. We can write it as a structure and it is as follows.
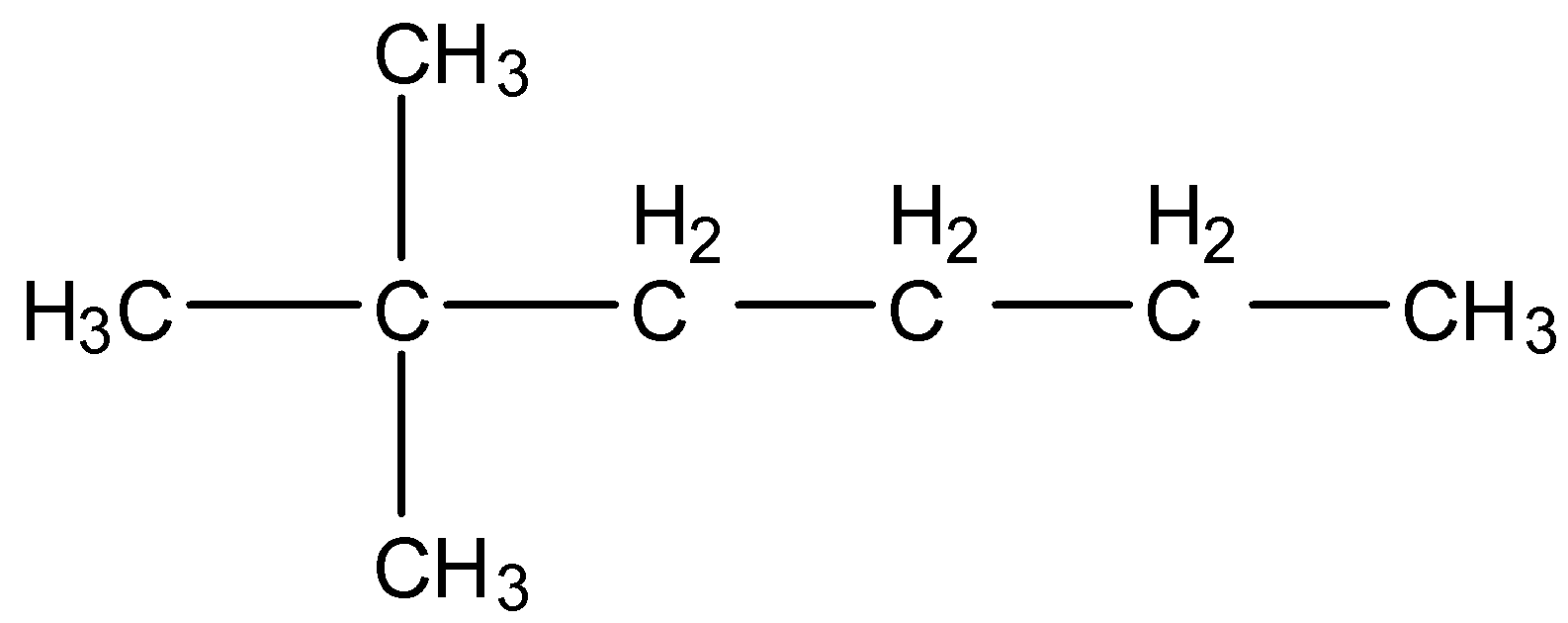
- The IUPAC name of the above structure is 2,2-dimethyl hexane.
- Coming to option B, $C{{H}_{3}}-CH(C{{H}_{3}})-CH(C{{H}_{3}})-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}$ . We can write it as a structure and it is as follows.
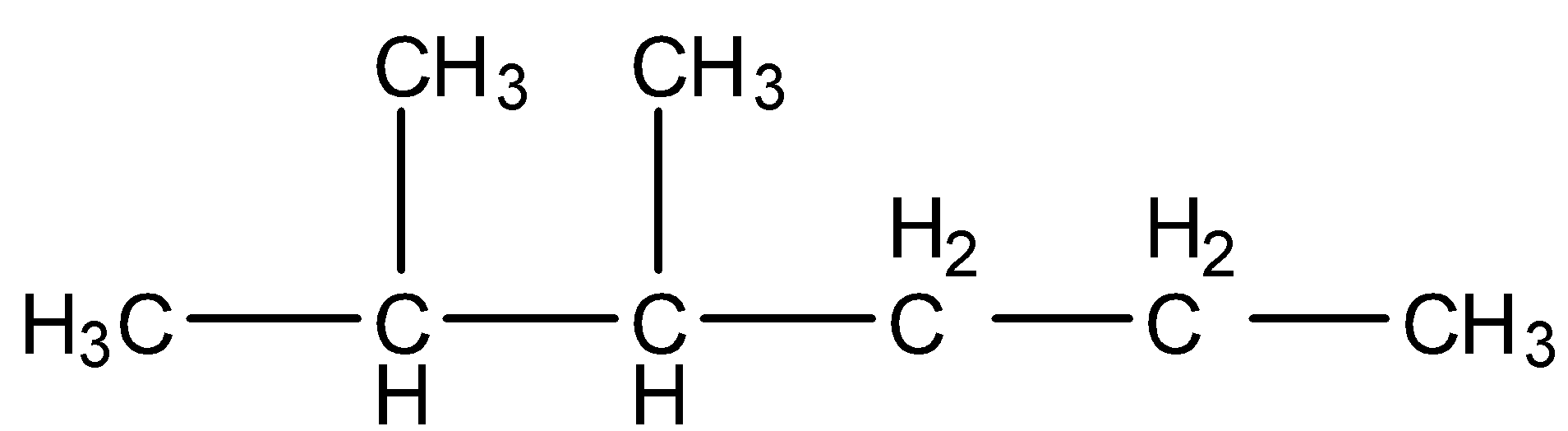
- The IUPAC name of the above structure is 2,3-dimethyl hexane.
- Coming to option C, $C{{H}_{3}}-CH(C{{H}_{3}})-C{{H}_{2}}-CH(C{{H}_{3}})-C{{H}_{2}}-C{{H}_{3}}$ . We can write it as a structure and it is as follows.
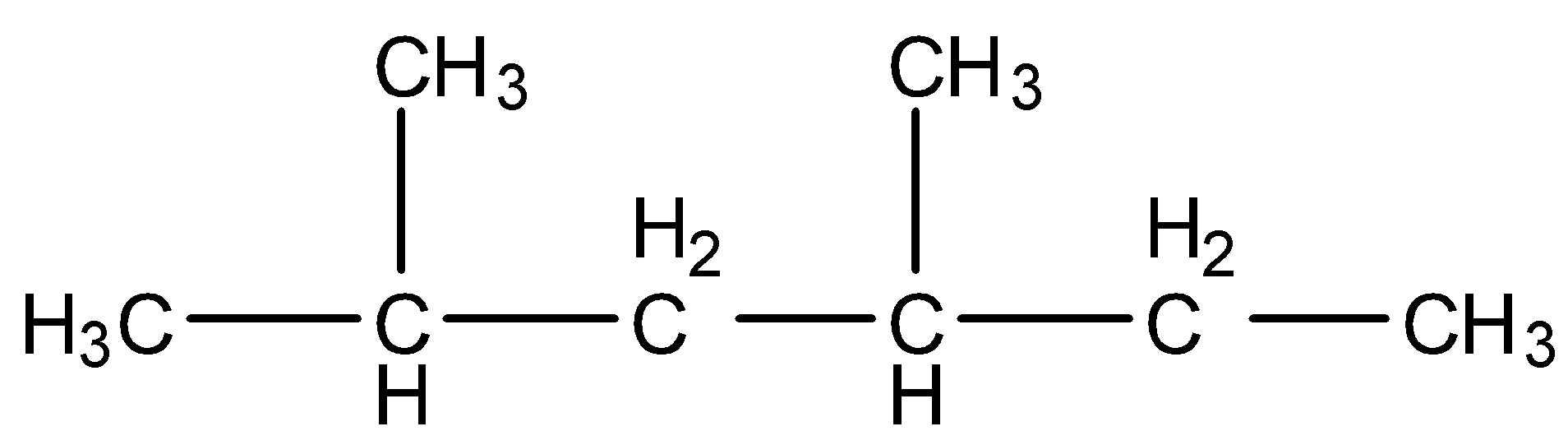
- The IUPAC name of the above structure is 2,4-dimethyl hexane.
- Coming to option D, $C{{H}_{3}}-CH(C{{H}_{3}})-C{{H}_{2}}-C{{H}_{2}}-CH(C{{H}_{3}})-C{{H}_{3}}$ . We can write it as a structure and it is as follows.
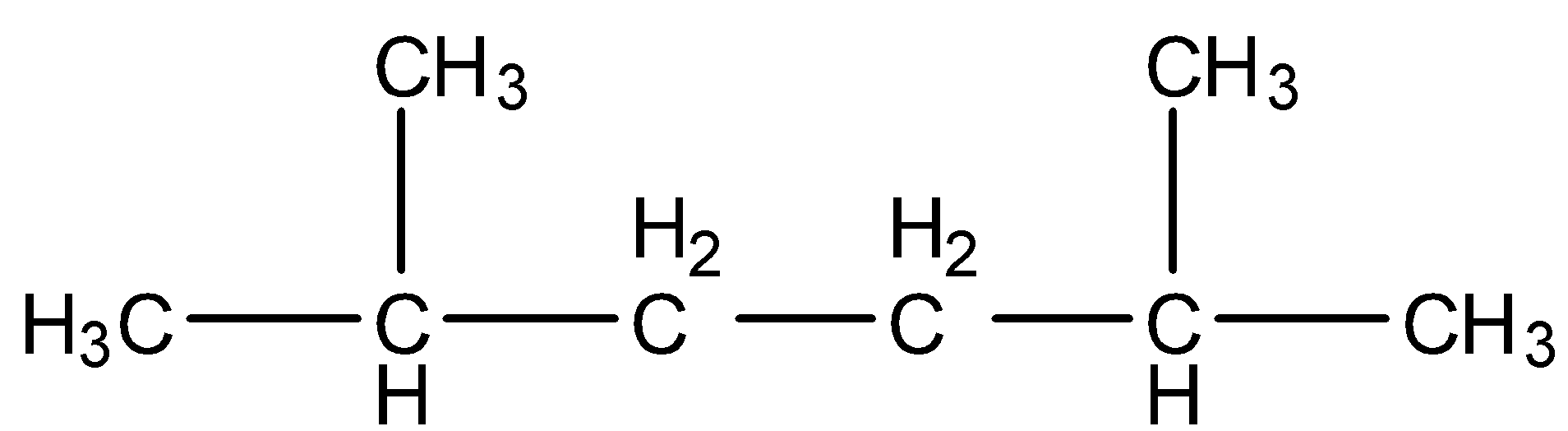
- The IUPAC name of the above structure is 2,5-dimethyl hexane.
- Therefore the structure of 2,4 –dimethyl hexane is $C{{H}_{3}}-CH(C{{H}_{3}})-C{{H}_{2}}-CH(C{{H}_{3}})-C{{H}_{2}}-C{{H}_{3}}$ . We can write it as a structure and it is as follows. The correct answer is option “C” .
Note: We should give lowest numbering to the highly substituted carbon first and the name at the end of the IUPAC name is going to decide by the presence of the number of carbons in the longest chain of the given organic compound. The name for the number of carbons in the chain
meth – one carbon
eth – two carbons
prop – three carbons
but – four carbons
pent – five carbons
hex – six carbons
hept – seven carbons
oct – eight carbons
non – nine carbons
deca – ten carbons.
Complete step by step answer:
- In the question it is given to identify the structure of the compound with the IUPAC name 2,4 –dimethylhexane among the given options.
- Coming to given options, option A, $C{{H}_{3}}-C{{(C{{H}_{3}})}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}$. We can write it as a structure and it is as follows.

- The IUPAC name of the above structure is 2,2-dimethyl hexane.
- Coming to option B, $C{{H}_{3}}-CH(C{{H}_{3}})-CH(C{{H}_{3}})-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}$ . We can write it as a structure and it is as follows.

- The IUPAC name of the above structure is 2,3-dimethyl hexane.
- Coming to option C, $C{{H}_{3}}-CH(C{{H}_{3}})-C{{H}_{2}}-CH(C{{H}_{3}})-C{{H}_{2}}-C{{H}_{3}}$ . We can write it as a structure and it is as follows.

- The IUPAC name of the above structure is 2,4-dimethyl hexane.
- Coming to option D, $C{{H}_{3}}-CH(C{{H}_{3}})-C{{H}_{2}}-C{{H}_{2}}-CH(C{{H}_{3}})-C{{H}_{3}}$ . We can write it as a structure and it is as follows.

- The IUPAC name of the above structure is 2,5-dimethyl hexane.
- Therefore the structure of 2,4 –dimethyl hexane is $C{{H}_{3}}-CH(C{{H}_{3}})-C{{H}_{2}}-CH(C{{H}_{3}})-C{{H}_{2}}-C{{H}_{3}}$ . We can write it as a structure and it is as follows. The correct answer is option “C” .
Note: We should give lowest numbering to the highly substituted carbon first and the name at the end of the IUPAC name is going to decide by the presence of the number of carbons in the longest chain of the given organic compound. The name for the number of carbons in the chain
meth – one carbon
eth – two carbons
prop – three carbons
but – four carbons
pent – five carbons
hex – six carbons
hept – seven carbons
oct – eight carbons
non – nine carbons
deca – ten carbons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




