
Which of the following have planar structure?
A. ${I_3}^ - $
B. $IC{l_3}$
C. $C{l_3}{O_6}$
D. $B{e_2}C{l_4}$
Answer
569.4k+ views
Hint: The planar structure of a compound depends on the plane on which the bonds of the compound are present. If all the bonds should be present in one plane only. It does not depend on the presence of lone pair of electrons which may be present in the compound
Complete step by step answer:
A compound is said to be planar if all the bonds of the compounds lie in one plane. It depends on the hybridisation of the compound. That is if the compound has the $sp$ hybridisation then it will be planar. Usually the $s{p^2},s{p^3},s{p^3}d$ and so on are all non planar.
If we consider the first compound mentioned in the options that is, ${I_3}^ - $ . we first need to find the number of electrons in the valence shell. Since it is a halogen, we know that the outermost shell will have $7$ electrons. Therefore the electronic configuration can be demonstrated as follows
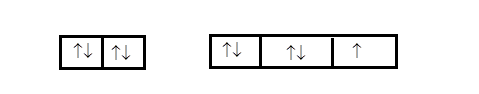
$5{s^2}$ $5{p^5}$
Therefore, we can see that the orbitals that contain fully filled orbitals can be considered as lone pairs. Since three orbitals are fully filled the central iodine will have three lone pairs.
Now we have two more iodine ions bonded to the central atom therefore, in order to prevent maximum repulsion the geometry of the compound will be trigonal bipyramidal. This is shown below:
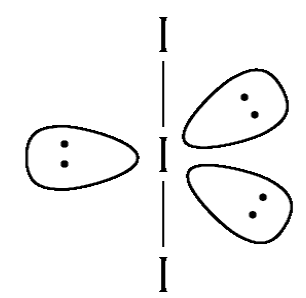
Therefore, we can see that the lone pair of electrons use the equatorial plane and the axial plane is occupied by the bond pairs. Therefore, the bond pairs are present in the same plane we can say ${I_3}^ - $ is planar.
The next option has iodine at the centre as well. Here the three chlorine atoms will combine with iodine. The iodine will have three bond pairs and two lone pairs and will therefore, have $s{p^3}d$ hybridisation. This will have the structure as shown below:
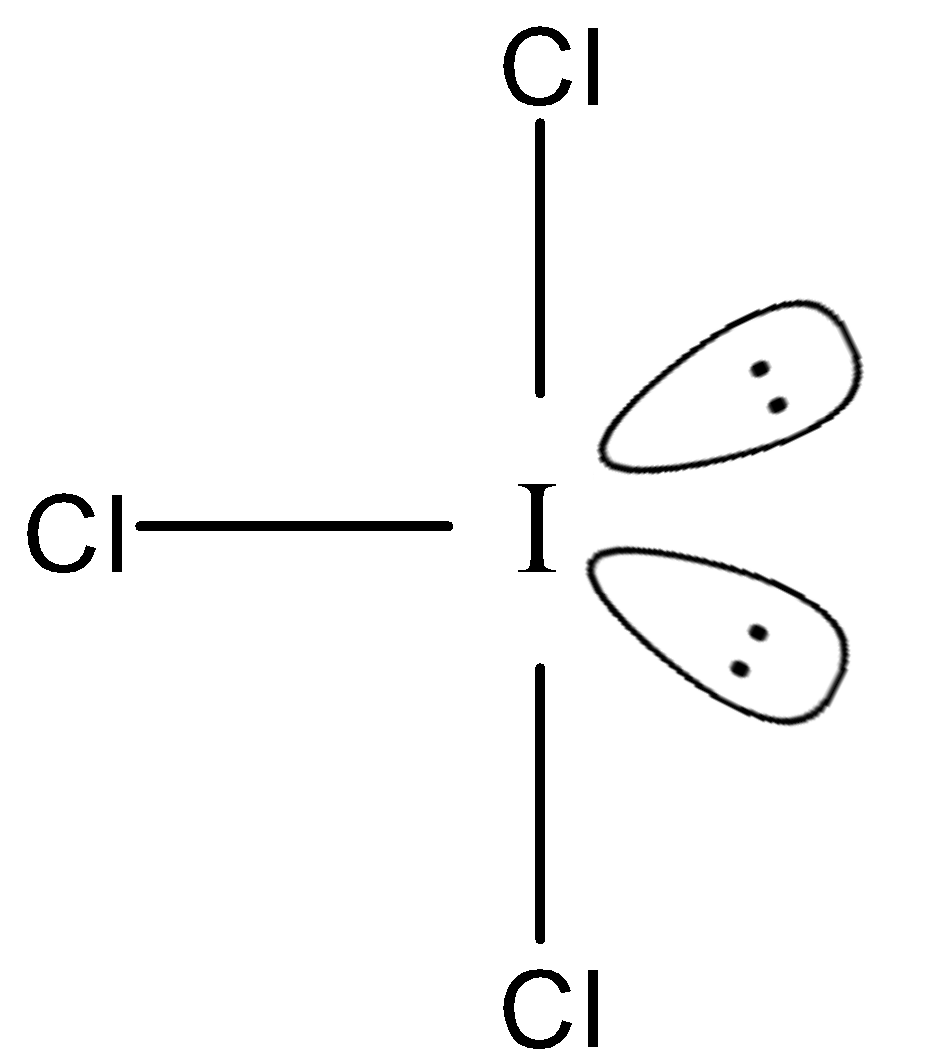
Therefore, the chlorine atoms occupy two different planes. Therefore, it will not be planar.
Option c consists of $C{l_3}{O_6}$ therefore, the chlorine will be attached to two oxygen atoms. But this will also not contain all its atoms in one plane. Therefore, it is non planar.
$B{e_2}C{l_4}$ is a dimer of $BeC{l_2}$ . $BeC{l_2}$ is $sp$ hybridised and will therefore be linear and also planar in nature. But the dimer that is, $B{e_2}C{l_4}$ is $s{p^3}$ hybridised. Therefore, this structure is not planar now.
Therefore, we can conclude by saying that the molecule that is planar among these is option A that is ${I_3}^ - $ .
So, the correct answer is Option A.
Note: Lone pairs have no effect when we consider the plane of the molecule. That is, a molecule is said to be planar if all the bond pairs are present in the same plane.
A lone pair is formed when an orbital of the central atom is completely filled.
Complete step by step answer:
A compound is said to be planar if all the bonds of the compounds lie in one plane. It depends on the hybridisation of the compound. That is if the compound has the $sp$ hybridisation then it will be planar. Usually the $s{p^2},s{p^3},s{p^3}d$ and so on are all non planar.
If we consider the first compound mentioned in the options that is, ${I_3}^ - $ . we first need to find the number of electrons in the valence shell. Since it is a halogen, we know that the outermost shell will have $7$ electrons. Therefore the electronic configuration can be demonstrated as follows

$5{s^2}$ $5{p^5}$
Therefore, we can see that the orbitals that contain fully filled orbitals can be considered as lone pairs. Since three orbitals are fully filled the central iodine will have three lone pairs.
Now we have two more iodine ions bonded to the central atom therefore, in order to prevent maximum repulsion the geometry of the compound will be trigonal bipyramidal. This is shown below:

Therefore, we can see that the lone pair of electrons use the equatorial plane and the axial plane is occupied by the bond pairs. Therefore, the bond pairs are present in the same plane we can say ${I_3}^ - $ is planar.
The next option has iodine at the centre as well. Here the three chlorine atoms will combine with iodine. The iodine will have three bond pairs and two lone pairs and will therefore, have $s{p^3}d$ hybridisation. This will have the structure as shown below:

Therefore, the chlorine atoms occupy two different planes. Therefore, it will not be planar.
Option c consists of $C{l_3}{O_6}$ therefore, the chlorine will be attached to two oxygen atoms. But this will also not contain all its atoms in one plane. Therefore, it is non planar.
$B{e_2}C{l_4}$ is a dimer of $BeC{l_2}$ . $BeC{l_2}$ is $sp$ hybridised and will therefore be linear and also planar in nature. But the dimer that is, $B{e_2}C{l_4}$ is $s{p^3}$ hybridised. Therefore, this structure is not planar now.
Therefore, we can conclude by saying that the molecule that is planar among these is option A that is ${I_3}^ - $ .
So, the correct answer is Option A.
Note: Lone pairs have no effect when we consider the plane of the molecule. That is, a molecule is said to be planar if all the bond pairs are present in the same plane.
A lone pair is formed when an orbital of the central atom is completely filled.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




