
Which of the following have least polarity in bond?
A)$H - F$
B) $H - Cl$
C) $H - O$
D) $H - S$
Answer
547.8k+ views
Hint: The bonding between atoms of similar electrical charge (cation or anion) the \[C{l_2},{H_2},{O_2}\] molecules and methane, Ethane etc. Organic compounds may be explained on its basis. Each gives for sharing for covalence. This electron is bonded such that it belongs to both the atoms. The covalence results in the formation of a molecule.
Complete step by step answer:
The sharing of electrons between the bonded atoms forming a compound is such that each atom attains its nearest noble gas configuration that is its outermost shell contains \[8\] electrons and only the valence shell of hydrogen consists of two electrons. Half of the electrons among shared pairs are given by one atom and the other half by the other atom. The shared electron pairs however belong to both the atoms and the shared electron pairs are at equal distances from the nuclei of the two atoms.
The bond formed by the sharing of electrons between two atoms is called the covalent bond. Does a covalent bond formed by the formation of electrons, pair between two items due to the sharing of the electrons. Since the molecules formed as a result of covalent bonding do not contain any charges they are called non polar or non-ionic.
The electronegativity of the elements are-
Hence option D is correct.
Additional information:
Types of covalent bond = A covalent bond is of two types-
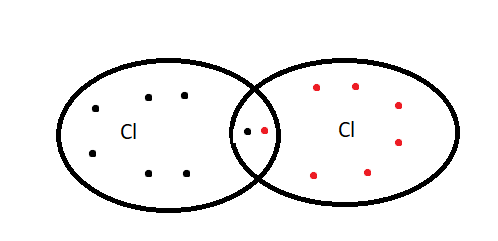
1.Nonpolar covalent bond covalent bond= In this type of covalent bond the shared electron pair between two atoms is situated at the centre, between the two atoms because both the atoms have similar electronegativity. This bond is formed between the nuclear atoms such items are neutral. Example \[{H_2},{\text{ C}}{l_2}\]
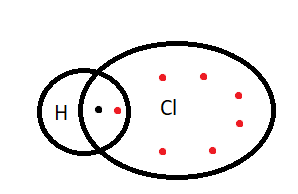
2. Polar covalent bond= When shared the electron pair in the covalent bond between the two atoms instead of being situated at the centre is attracted more towards an atom then such a bond is called the polar covalent bond such a bond is formed between two such items which have differences in their electronegativity. The larger is the difference between the electronegativity of two atoms the more polar is the bond. The atom towards which an electron pair is attracted more attains the negative charges and the atoms from which it is farther attains the positive charges. Such molecules possess a dipole moment that is a molecule that possesses positive and negative poles. Such compounds are called polar covalent compounds. For example \[HCl,{\text{ }}HBr\]
That is why such molecules undergo ionization.
Electronegativity= The tendency of an atom to attract an electron to itself is called electronegativity. Electronegativity is represented by the Greek letter Ki ($\chi $). When two elements with different electronegativity form covalent bonds then the resulting compound is Polar. It means that the electron pair is attracted towards the atoms which is more electronegative.
Note: We have to remember that which element has the least polarity as this can define the least polarity of the pair of elements. The less the negativity the less the polarity. Polar covalent bonds show the polarity in the compound.
Complete step by step answer:
The sharing of electrons between the bonded atoms forming a compound is such that each atom attains its nearest noble gas configuration that is its outermost shell contains \[8\] electrons and only the valence shell of hydrogen consists of two electrons. Half of the electrons among shared pairs are given by one atom and the other half by the other atom. The shared electron pairs however belong to both the atoms and the shared electron pairs are at equal distances from the nuclei of the two atoms.
The bond formed by the sharing of electrons between two atoms is called the covalent bond. Does a covalent bond formed by the formation of electrons, pair between two items due to the sharing of the electrons. Since the molecules formed as a result of covalent bonding do not contain any charges they are called non polar or non-ionic.
The electronegativity of the elements are-
| Element | electronegativity |
| \[\begin{array}{*{20}{l}} F \end{array}\] | \[\begin{array}{*{20}{l}} {4.0} \end{array}\] |
| \[\begin{array}{*{20}{l}} O \end{array}\] | \[\begin{array}{*{20}{l}} {3.5} \end{array}\] |
| \[N\] | \[\begin{array}{*{20}{l}} {3.0} \end{array}\] |
| \[\begin{array}{*{20}{l}} {Cl} \end{array}\] | \[\begin{array}{*{20}{l}} {3.0} \end{array}\] |
| \[\begin{array}{*{20}{l}} C \end{array}\] | \[\begin{array}{*{20}{l}} {2.5} \end{array}\] |
| \[\begin{array}{*{20}{l}} H \end{array}\] | \[\begin{array}{*{20}{l}} {2.1} \end{array}\] |
Hence option D is correct.
Additional information:
Types of covalent bond = A covalent bond is of two types-
1.Nonpolar covalent bond covalent bond= In this type of covalent bond the shared electron pair between two atoms is situated at the centre, between the two atoms because both the atoms have similar electronegativity. This bond is formed between the nuclear atoms such items are neutral. Example \[{H_2},{\text{ C}}{l_2}\]

2. Polar covalent bond= When shared the electron pair in the covalent bond between the two atoms instead of being situated at the centre is attracted more towards an atom then such a bond is called the polar covalent bond such a bond is formed between two such items which have differences in their electronegativity. The larger is the difference between the electronegativity of two atoms the more polar is the bond. The atom towards which an electron pair is attracted more attains the negative charges and the atoms from which it is farther attains the positive charges. Such molecules possess a dipole moment that is a molecule that possesses positive and negative poles. Such compounds are called polar covalent compounds. For example \[HCl,{\text{ }}HBr\]
That is why such molecules undergo ionization.

Electronegativity= The tendency of an atom to attract an electron to itself is called electronegativity. Electronegativity is represented by the Greek letter Ki ($\chi $). When two elements with different electronegativity form covalent bonds then the resulting compound is Polar. It means that the electron pair is attracted towards the atoms which is more electronegative.
Note: We have to remember that which element has the least polarity as this can define the least polarity of the pair of elements. The less the negativity the less the polarity. Polar covalent bonds show the polarity in the compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




