
Which of the following has the highest value of dipole moments?
A. ${\text{C}}{{\text{O}}_{\text{2}}}$
B. ${\text{S}}{{\text{O}}_{\text{2}}}$
C. ${\text{S}}{{\text{O}}_{\text{3}}}$
D. ${\text{CC}}{{\text{l}}_{\text{4}}}$
Answer
565.8k+ views
Hint: To answer this question we should know what a dipole moment is and how to determine the dipole moment. The dipole moment is the direction of the electric charge that arises due to the separation of charge. We will determine the dipole moment by drawing the geometry of the given molecules. After drawing the geometry we will check the direction of the polarities of the bonds. If the direction is opposite then the molecule will have zero dipole moment. If the direction is not opposite then the molecule will have a dipole moment.
Complete step-by-step answer:Dipole moment arises due to the difference in electronegative of the bonded atoms. The atom has high electronegativity attracts the bonded electrons towards itself, so it gets a partial negative charge and another atom having less electronegativity gets the partial positive charge.
The direction of dipole moment is shown by an arrow , pointing towards the high electronegative atom.
The structure of the ${\text{C}}{{\text{O}}_{\text{2}}}$ is as follows:
The hybridization of the ${\text{C}}{{\text{O}}_{\text{2}}}$ is ${\text{sp}}$ so, the geometry is linear.
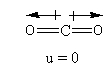
The oxygen is more electronegative than carbon so, the direction of the dipole moment of the C-O bond will be towards the oxygen. As the structure is linear so, both C-O bonds are so, the dipole moment of both C-O bonds cancel exactly, so that carbon dioxide has a zero total permanent dipole moment thus ${\text{C}}{{\text{O}}_{\text{2}}}$ will not show dipole-dipole interactions.
The structure of the ${\text{S}}{{\text{O}}_{\text{2}}}$ is as follows:
The hybridization of the ${\text{S}}{{\text{O}}_{\text{2}}}$ is ${\text{s}}{{\text{p}}^2}$ so, the geometry is a trigonal planar but it has two bond pair and one lone pair so, its shape is bent.
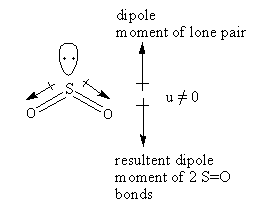
The oxygen is more electronegative than sulphur so, the direction of the dipole moment of the S-O bond will be towards the oxygen. As the structure is bent so, two S-O bonds are almost in the same direction and the lone pair is in the opposite direction. As lone pair and bond pair do not have the same magnitude of charge so, the dipole moment of S-O bonds and lone pair does not get cancel exactly so that sulphur dioxide has a magnitude of total permanent dipole moment thus ${\text{S}}{{\text{O}}_2}$ will show dipole-dipole interactions.
The structure of the ${\text{S}}{{\text{O}}_3}$ is as follows:
The hybridization of the ${\text{S}}{{\text{O}}_3}$ is ${\text{s}}{{\text{p}}^2}$ so, the geometry is a trigonal planar.
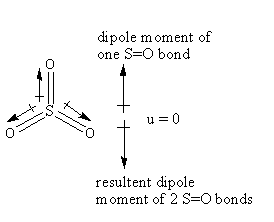
The oxygen is more electronegative than sulphur so, the direction of the dipole moment of the S-O bond will be towards the oxygen. As the structure is a trigonal planar, two S-O bonds are almost in the same direction and one S-O bond is in the opposite direction. As the resultant polarity of S-O bonds is opposite so, the dipole moment of all S-O bonds cancels out by each other exactly, so that sulphur trioxide has a zero total permanent dipole moment thus ${\text{S}}{{\text{O}}_3}$ will not show dipole-dipole interactions.
The structure of the ${\text{CC}}{{\text{l}}_4}$ is as follows:
The hybridization of the ${\text{CC}}{{\text{l}}_4}$ is ${\text{s}}{{\text{p}}^3}$ so, the geometry is tetrahedral.
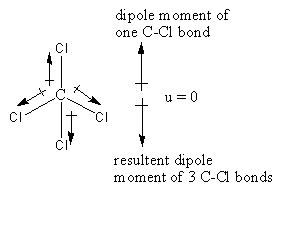
The chlorine is more electronegative than carbon so, the direction of the dipole moment of the C-Cl bond will be towards the chlorine. As the structure is tetrahedral so, three C-Cl bonds are almost in the same direction and one C-Cl bond is in the opposite direction. As the resultant polarity of three C-Cl bonds is in the same direction and polarity of the fourth bond is in opposite direction so, the dipole moment of all C-Cl bonds cancel out by each other exactly, so that carbon tetrachloride has a zero total permanent dipole moment thus ${\text{CC}}{{\text{l}}_4}$ will not show dipole-dipole interactions.
So, ${\text{S}}{{\text{O}}_{\text{2}}}$ has the highest value of dipole moments.
Therefore, option (B),${\text{S}}{{\text{O}}_{\text{2}}}$ is correct.
Note:Dipole moment can occur between two ions in an ionic bond or between atoms in a covalent bond. As the dipole moment has both magnitude and direction, so it is a vector quantity. The formula to calculate the magnitude of dipole moment is as follows:
$\mu = \,{\text{q}}{\text{.D}}$
Where,
$\mu \,$ is the dipole moment.
${\text{q}}$ is the magnitude of charge.
${\text{D}}$ is the distance between two chemical bonded atoms.
The perfect geometry having the same surrounding atoms always has zero dipole moment such as perfect tetrahedral, octahedral, and linear, planner, etc.
Complete step-by-step answer:Dipole moment arises due to the difference in electronegative of the bonded atoms. The atom has high electronegativity attracts the bonded electrons towards itself, so it gets a partial negative charge and another atom having less electronegativity gets the partial positive charge.
The direction of dipole moment is shown by an arrow , pointing towards the high electronegative atom.
The structure of the ${\text{C}}{{\text{O}}_{\text{2}}}$ is as follows:
The hybridization of the ${\text{C}}{{\text{O}}_{\text{2}}}$ is ${\text{sp}}$ so, the geometry is linear.
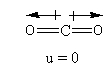
The oxygen is more electronegative than carbon so, the direction of the dipole moment of the C-O bond will be towards the oxygen. As the structure is linear so, both C-O bonds are so, the dipole moment of both C-O bonds cancel exactly, so that carbon dioxide has a zero total permanent dipole moment thus ${\text{C}}{{\text{O}}_{\text{2}}}$ will not show dipole-dipole interactions.
The structure of the ${\text{S}}{{\text{O}}_{\text{2}}}$ is as follows:
The hybridization of the ${\text{S}}{{\text{O}}_{\text{2}}}$ is ${\text{s}}{{\text{p}}^2}$ so, the geometry is a trigonal planar but it has two bond pair and one lone pair so, its shape is bent.

The oxygen is more electronegative than sulphur so, the direction of the dipole moment of the S-O bond will be towards the oxygen. As the structure is bent so, two S-O bonds are almost in the same direction and the lone pair is in the opposite direction. As lone pair and bond pair do not have the same magnitude of charge so, the dipole moment of S-O bonds and lone pair does not get cancel exactly so that sulphur dioxide has a magnitude of total permanent dipole moment thus ${\text{S}}{{\text{O}}_2}$ will show dipole-dipole interactions.
The structure of the ${\text{S}}{{\text{O}}_3}$ is as follows:
The hybridization of the ${\text{S}}{{\text{O}}_3}$ is ${\text{s}}{{\text{p}}^2}$ so, the geometry is a trigonal planar.

The oxygen is more electronegative than sulphur so, the direction of the dipole moment of the S-O bond will be towards the oxygen. As the structure is a trigonal planar, two S-O bonds are almost in the same direction and one S-O bond is in the opposite direction. As the resultant polarity of S-O bonds is opposite so, the dipole moment of all S-O bonds cancels out by each other exactly, so that sulphur trioxide has a zero total permanent dipole moment thus ${\text{S}}{{\text{O}}_3}$ will not show dipole-dipole interactions.
The structure of the ${\text{CC}}{{\text{l}}_4}$ is as follows:
The hybridization of the ${\text{CC}}{{\text{l}}_4}$ is ${\text{s}}{{\text{p}}^3}$ so, the geometry is tetrahedral.

The chlorine is more electronegative than carbon so, the direction of the dipole moment of the C-Cl bond will be towards the chlorine. As the structure is tetrahedral so, three C-Cl bonds are almost in the same direction and one C-Cl bond is in the opposite direction. As the resultant polarity of three C-Cl bonds is in the same direction and polarity of the fourth bond is in opposite direction so, the dipole moment of all C-Cl bonds cancel out by each other exactly, so that carbon tetrachloride has a zero total permanent dipole moment thus ${\text{CC}}{{\text{l}}_4}$ will not show dipole-dipole interactions.
So, ${\text{S}}{{\text{O}}_{\text{2}}}$ has the highest value of dipole moments.
Therefore, option (B),${\text{S}}{{\text{O}}_{\text{2}}}$ is correct.
Note:Dipole moment can occur between two ions in an ionic bond or between atoms in a covalent bond. As the dipole moment has both magnitude and direction, so it is a vector quantity. The formula to calculate the magnitude of dipole moment is as follows:
$\mu = \,{\text{q}}{\text{.D}}$
Where,
$\mu \,$ is the dipole moment.
${\text{q}}$ is the magnitude of charge.
${\text{D}}$ is the distance between two chemical bonded atoms.
The perfect geometry having the same surrounding atoms always has zero dipole moment such as perfect tetrahedral, octahedral, and linear, planner, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




