
Which of the following compounds react with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] and \[{\text{HCl}}\] at \[{\text{0 - 4}}^\circ {\text{C}}\] to give alcohol/phenol?
A. \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]
B. \[{{\text{C}}_2}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]
C. \[{\text{C}}{{\text{H}}_3}{\text{NHC}}{{\text{H}}_3}\]
D. \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}\]
Answer
573.9k+ views
Hint: The reagent \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] and \[{\text{HCl}}\] used to distinguish primary, secondary and tertiary amines. Only primary aliphatic amines react with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] and \[{\text{HCl}}\] at \[{\text{0 - 4}}^\circ {\text{C}}\] . The product of the reaction is alcohol.
Complete Step by step answer: The reagent given to us is \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] and \[{\text{HCl}}\] and the reaction condition is \[{\text{0 - 4}}^\circ {\text{C}}\]. Only primary aliphatic amines react with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] and \[{\text{HCl}}\] at \[{\text{0 - 4}}^\circ {\text{C}}\] and give alcohol as the product.
The amine given in option A is \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]. Its structure is as follows:
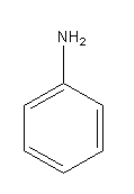
It is aromatic amine so it will not give phenol after reacting with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] in \[{\text{HCl}}\] at \[{\text{0 - 4}}^\circ {\text{C}}\].
Thus, option (A) \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\] is incorrect.
The amine given in option B is\[{{\text{C}}_2}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]. Its structure is as follows:
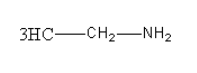
It is a primary aliphatic amine so it will give alcohol after reacting with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] in \[{\text{HCl}}\] at \[{\text{0 - 4}}^\circ {\text{C}}\].
So, option (B) \[{{\text{C}}_2}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]is correct.
The amine given in option C is \[{\text{C}}{{\text{H}}_3}{\text{NHC}}{{\text{H}}_3}\]. Its structure is as follows:
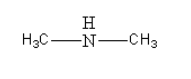
It is secondary aliphatic amine so it will not give alcohol after reacting with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] in \[{\text{HCl}}\] at \[{\text{0 - 4}}^\circ {\text{C}}\].
Thus, option (C) \[{\text{C}}{{\text{H}}_3}{\text{NHC}}{{\text{H}}_3}\] is incorrect.
The amine given in option D is \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}\]. Its structure is as follows:
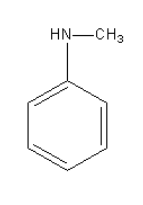
It is aromatic secondary amine so it will not give phenol after reacting with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] in \[{\text{HCl}}\] at \[{\text{0 - 4}}^\circ {\text{C}}\].
Thus, option (D) \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}\] is incorrect.
Hence, option (B) \[{{\text{C}}_2}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]is the correct answer.
Note: Aliphatic primary amines react with\[{\text{NaN}}{{\text{O}}_{\text{2}}}\] in \[{\text{HCl}}\] at \[{\text{0 - 4}}^\circ {\text{C}}\]and undergo diazotization to form alkane diazonium salt, which however being unstable decomposes to form a mixture of alcohols, alkene with the liberation of \[{{\text{N}}_{\text{2}}}\] gas. Secondary amines react with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] in \[{\text{HCl}}\] to form N-nitrosamines.
Complete Step by step answer: The reagent given to us is \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] and \[{\text{HCl}}\] and the reaction condition is \[{\text{0 - 4}}^\circ {\text{C}}\]. Only primary aliphatic amines react with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] and \[{\text{HCl}}\] at \[{\text{0 - 4}}^\circ {\text{C}}\] and give alcohol as the product.
The amine given in option A is \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]. Its structure is as follows:

It is aromatic amine so it will not give phenol after reacting with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] in \[{\text{HCl}}\] at \[{\text{0 - 4}}^\circ {\text{C}}\].
Thus, option (A) \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\] is incorrect.
The amine given in option B is\[{{\text{C}}_2}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]. Its structure is as follows:

It is a primary aliphatic amine so it will give alcohol after reacting with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] in \[{\text{HCl}}\] at \[{\text{0 - 4}}^\circ {\text{C}}\].
So, option (B) \[{{\text{C}}_2}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]is correct.
The amine given in option C is \[{\text{C}}{{\text{H}}_3}{\text{NHC}}{{\text{H}}_3}\]. Its structure is as follows:

It is secondary aliphatic amine so it will not give alcohol after reacting with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] in \[{\text{HCl}}\] at \[{\text{0 - 4}}^\circ {\text{C}}\].
Thus, option (C) \[{\text{C}}{{\text{H}}_3}{\text{NHC}}{{\text{H}}_3}\] is incorrect.
The amine given in option D is \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}\]. Its structure is as follows:

It is aromatic secondary amine so it will not give phenol after reacting with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] in \[{\text{HCl}}\] at \[{\text{0 - 4}}^\circ {\text{C}}\].
Thus, option (D) \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{NHC}}{{\text{H}}_{\text{3}}}\] is incorrect.
Hence, option (B) \[{{\text{C}}_2}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]is the correct answer.
Note: Aliphatic primary amines react with\[{\text{NaN}}{{\text{O}}_{\text{2}}}\] in \[{\text{HCl}}\] at \[{\text{0 - 4}}^\circ {\text{C}}\]and undergo diazotization to form alkane diazonium salt, which however being unstable decomposes to form a mixture of alcohols, alkene with the liberation of \[{{\text{N}}_{\text{2}}}\] gas. Secondary amines react with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] in \[{\text{HCl}}\] to form N-nitrosamines.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




