
Which of the following compounds have all four types (${{1}^{\circ }},{{2}^{\circ }},{{3}^{\circ }},{{4}^{\circ }}$) of carbon atoms?
[A] 2,3,4-Trimethylpentane
[B] Neo pentane
[C] 2,2,4-Trimethylpentane
[D] None of the above
Answer
597k+ views
Hint: We can find the types of carbons in a compound by drawing their structures. Here, all the options have three methyl groups attached to the pentane .The pentane compound which will have two methyl groups attached to the same carbon and one methyl at any other position other than the same or consecutive carbon atom will have all four kinds of carbon atoms.
Complete answer: ${{1}^{\circ }}$Or primary carbon is the carbon atom which is attached to only one other carbon atom.
${{2}^{\circ }}$Or secondary carbon is the carbon atom which is attached to two other carbon atoms.
${{3}^{\circ }}$Or tertiary carbon is the carbon atom which is attached to three other carbon atoms.
${{4}^{\circ }}$Or quaternary carbon is the carbon atom which is attached to four other carbon atoms.
To find out the types of carbon atoms in the given compounds, we need to draw their structures.
In option [A] we have 2,3,4-Trimethylpentane. We can draw this as-
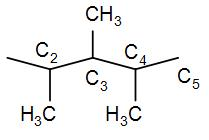
As we can see from the structure, it has-
-Two primary carbons C5 and C1 position.
-Three tertiary carbons at C2,C3 and C4 position.
It does not have secondary and quaternary carbon atoms. Therefore, it is not the correct answer.
In option [B] we have, Neo pentane
Neo pentane is${{C}_{5}}{{H}_{12}}$. Its structure is-
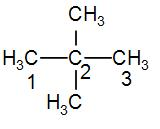 As we can see from the structure, neopentane has-
As we can see from the structure, neopentane has-
-Two primary carbons at the first and third carbon positions
-One quaternary carbon at C2 position.
It has no secondary or tertiary carbon atoms.
Now, in the option[C] we have, 2,2,4-Trimethylpentane
Structure of 2,2,4-Trimethylpentane is-
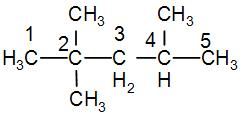 As we can see from the structure, it has-
-Two primary carbons at first and fifth carbon position.
As we can see from the structure, it has-
-Two primary carbons at first and fifth carbon position.
-One secondary carbon at the third carbon position.
-One tertiary carbon at the fourth carbon position.
-One quaternary carbon at the second carbon position.
It has all four kinds of carbon atoms.
Therefore, the correct answer is [C] 2,2,4-Trimethylpentane.
Note: To find out the number of primary, secondary, tertiary and quaternary carbons in a compound, we need to know the structure of the compound. Once we draw the compounds, we can easily count and find out the type of carbon atom.
Complete answer: ${{1}^{\circ }}$Or primary carbon is the carbon atom which is attached to only one other carbon atom.
${{2}^{\circ }}$Or secondary carbon is the carbon atom which is attached to two other carbon atoms.
${{3}^{\circ }}$Or tertiary carbon is the carbon atom which is attached to three other carbon atoms.
${{4}^{\circ }}$Or quaternary carbon is the carbon atom which is attached to four other carbon atoms.
To find out the types of carbon atoms in the given compounds, we need to draw their structures.
In option [A] we have 2,3,4-Trimethylpentane. We can draw this as-

As we can see from the structure, it has-
-Two primary carbons C5 and C1 position.
-Three tertiary carbons at C2,C3 and C4 position.
It does not have secondary and quaternary carbon atoms. Therefore, it is not the correct answer.
In option [B] we have, Neo pentane
Neo pentane is${{C}_{5}}{{H}_{12}}$. Its structure is-

-Two primary carbons at the first and third carbon positions
-One quaternary carbon at C2 position.
It has no secondary or tertiary carbon atoms.
Now, in the option[C] we have, 2,2,4-Trimethylpentane
Structure of 2,2,4-Trimethylpentane is-

-One secondary carbon at the third carbon position.
-One tertiary carbon at the fourth carbon position.
-One quaternary carbon at the second carbon position.
It has all four kinds of carbon atoms.
Therefore, the correct answer is [C] 2,2,4-Trimethylpentane.
Note: To find out the number of primary, secondary, tertiary and quaternary carbons in a compound, we need to know the structure of the compound. Once we draw the compounds, we can easily count and find out the type of carbon atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




