
Which of the following compounds have a number of $p\pi - p\pi $ bonds equal to $p\pi - d\pi $ bonds.
(A) $S{O_3}$
(B) $S{O_2}$
(C) $SOC{l_2}$
(D) $S{O_2}C{l_2}$
Answer
559.2k+ views
Hint: A covalent bond is a chemical bond formed by sharing of electron pairs. The sharing of electrons pairs is done by overlapping of orbitals and the side-to-side overlapping of p orbital with p orbital or p orbital with d orbital of two atoms gives $p\pi - p\pi $ and $p\pi - d\pi $ bonds. Sigma bonds are stronger than pi bonds.
Complete step by step answer:
Covalent bonds are chemical bonds which bond different atoms by sharing their electron pairs with each other. It is also known as molecular bonds. Pi bonds (π) are covalent bonds which are formed by the lateral overlapping of two lobes of an orbital of one atom on two lobes of an orbital of another atom. The bonded electrons by pi bond are called pi electrons. When the p orbital of one atom overlap side-to-side with the p orbital of another atom then this result in the formation of p π-p π bond, like $
C = O \\
\\
\\
$ . Similarly when d orbital overlaps side-to-side with the p orbital they form ap π-d π bond.
So, (A) $S{O_3}$ has $sp^2$ hybridization and no lone pair.
The excited state of $S{O_3}$ shows 2 electrons in d orbital and thus, those orbitals overlap with p orbital forming $2{{ }}p\pi - d\pi $ bonds and ${{1 }}p\pi - p\pi $ bond
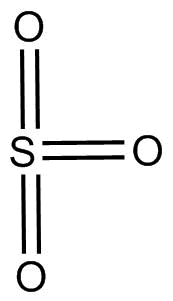

(B) $S{O_2}$ has sp2 hybridization and one lone pair is present over sulphur
The excited state of $S{O_2}$ show one electron in its d orbital which overlaps with the p orbital side-to-side and thus forms ${{1 }}p\pi - d\pi $ bond and ${{1 }}p\pi - d\pi $
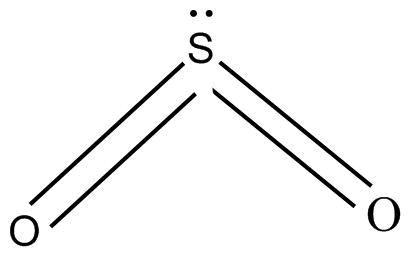
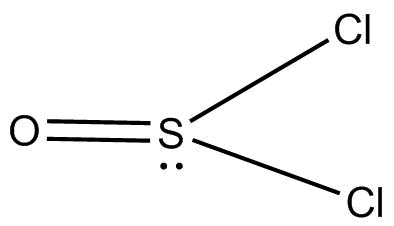
(C) $SOC{l_2}$ has S as a central atom with one lone pair double bonded oxygen atom and two chlorine atoms ${O = S}$ . The double bond contains ${{1 }}p\pi - d\pi $ bond.
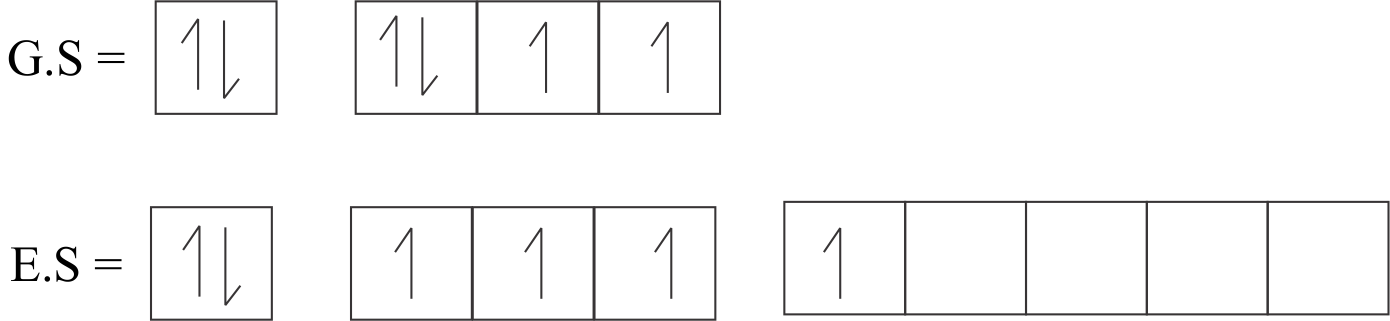
Sulphur has a hybridization of $[Ne]3{s^2}3{p^4}$:
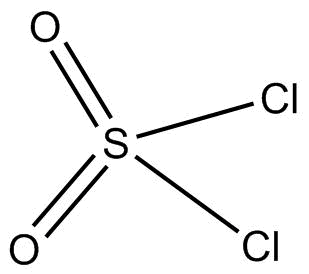
(D) $S{O_2}C{l_2}$ has sulphur as their central atom which is double bonded with two oxygen atoms and single bonded with two chlorine atoms. It has a tetrahedral geometry and it is therefore sp3 hybridized. It forms only $2{{ }}p\pi - d\pi $ bonds.
Thus, (B) $S{O_2}$ has ${{1 }}p\pi - d\pi $ and ${{1 }}p\pi - p\pi $.
Note: Back bonding is a type of resonance showcased by many compounds which increases their stability. It affects the overall dipole moment and the hybridization of a compound. There are two types where one is central atom to side atom and the other is other atom to side atom.
Complete step by step answer:
Covalent bonds are chemical bonds which bond different atoms by sharing their electron pairs with each other. It is also known as molecular bonds. Pi bonds (π) are covalent bonds which are formed by the lateral overlapping of two lobes of an orbital of one atom on two lobes of an orbital of another atom. The bonded electrons by pi bond are called pi electrons. When the p orbital of one atom overlap side-to-side with the p orbital of another atom then this result in the formation of p π-p π bond, like $
C = O \\
\\
\\
$ . Similarly when d orbital overlaps side-to-side with the p orbital they form ap π-d π bond.
So, (A) $S{O_3}$ has $sp^2$ hybridization and no lone pair.
The excited state of $S{O_3}$ shows 2 electrons in d orbital and thus, those orbitals overlap with p orbital forming $2{{ }}p\pi - d\pi $ bonds and ${{1 }}p\pi - p\pi $ bond


(B) $S{O_2}$ has sp2 hybridization and one lone pair is present over sulphur
The excited state of $S{O_2}$ show one electron in its d orbital which overlaps with the p orbital side-to-side and thus forms ${{1 }}p\pi - d\pi $ bond and ${{1 }}p\pi - d\pi $


(C) $SOC{l_2}$ has S as a central atom with one lone pair double bonded oxygen atom and two chlorine atoms ${O = S}$ . The double bond contains ${{1 }}p\pi - d\pi $ bond.
Sulphur has a hybridization of $[Ne]3{s^2}3{p^4}$:

(D) $S{O_2}C{l_2}$ has sulphur as their central atom which is double bonded with two oxygen atoms and single bonded with two chlorine atoms. It has a tetrahedral geometry and it is therefore sp3 hybridized. It forms only $2{{ }}p\pi - d\pi $ bonds.

Thus, (B) $S{O_2}$ has ${{1 }}p\pi - d\pi $ and ${{1 }}p\pi - p\pi $.
Note: Back bonding is a type of resonance showcased by many compounds which increases their stability. It affects the overall dipole moment and the hybridization of a compound. There are two types where one is central atom to side atom and the other is other atom to side atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




