
Which of the following compounds can exhibit tautomerism?

A) II and III
B) I, II and III
C) I and II
D) I and III

Answer
527.1k+ views
Hint: You first should know what tautomerism is. Tautomerism is an isomerism in which the isomers are readily interchangeable and are in dynamic equilibrium with each other. It is shown by the compounds which have an acidic $\alpha - hydrogen$. Keto compounds show tautomerism. Recall the keto-enol tautomerism.
Complete answer:
Tautomerism is a functional isomerism wherein the two structures which are isomers readily interconvert to each other and exist in dynamic equilibrium with each other. This reaction results in the relocation of proton (hydrogen atom) and the two structural isomers are called tautomers. Now, the two conditions for tautomerism are:
1. Compounds must have an electron withdrawing atom or group i.e., atom which is more electronegative than carbon and has the tendency to accept the hydrogen atom (as tautomerism involves migration of hydrogen atom).
2. There must be a carbon linked to that electron withdrawing atom and further this carbon is linked to another carbon having a hydrogen atom. This hydrogen is referred to as acidic $\alpha - hydrogen$.
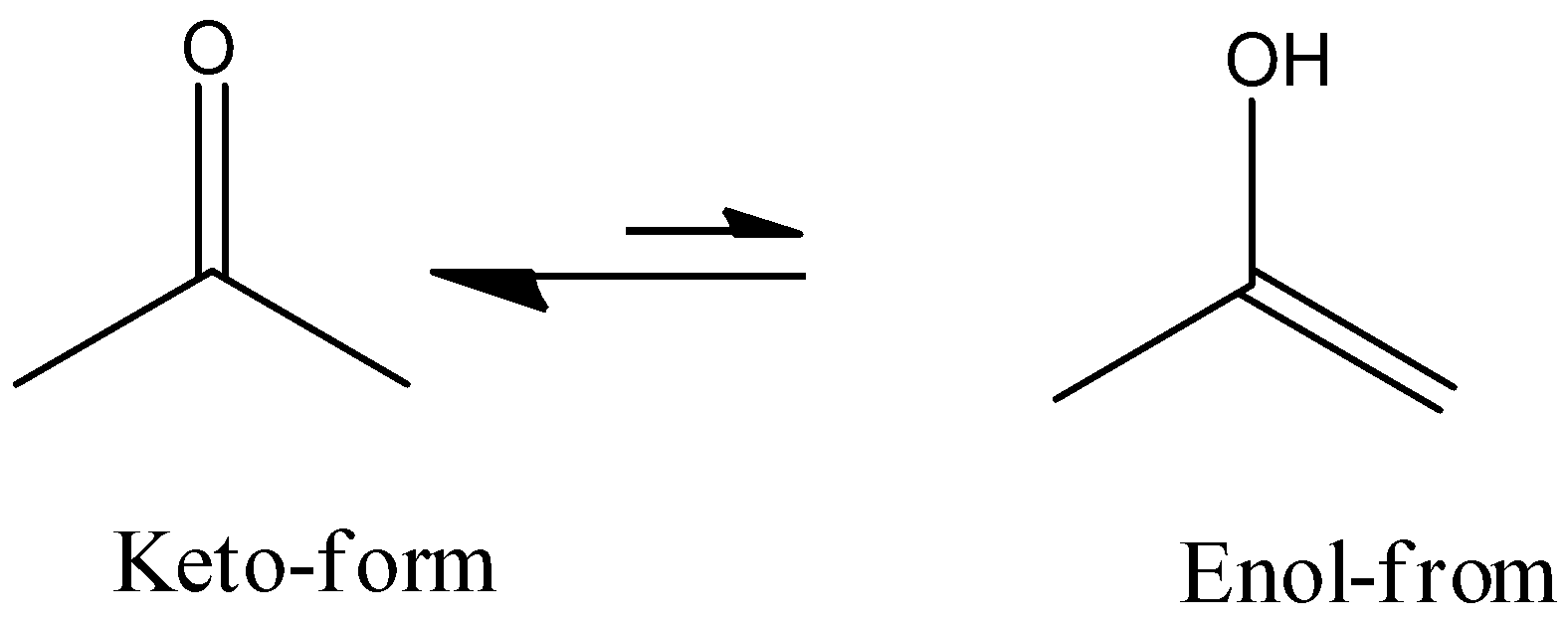
Keto compounds fulfill these conditions and the keto-enol tautomeric pair is one of the most common tautomeric pairs. Keto-enol tautomerism can be generally represented as:

Now, the given compounds are:
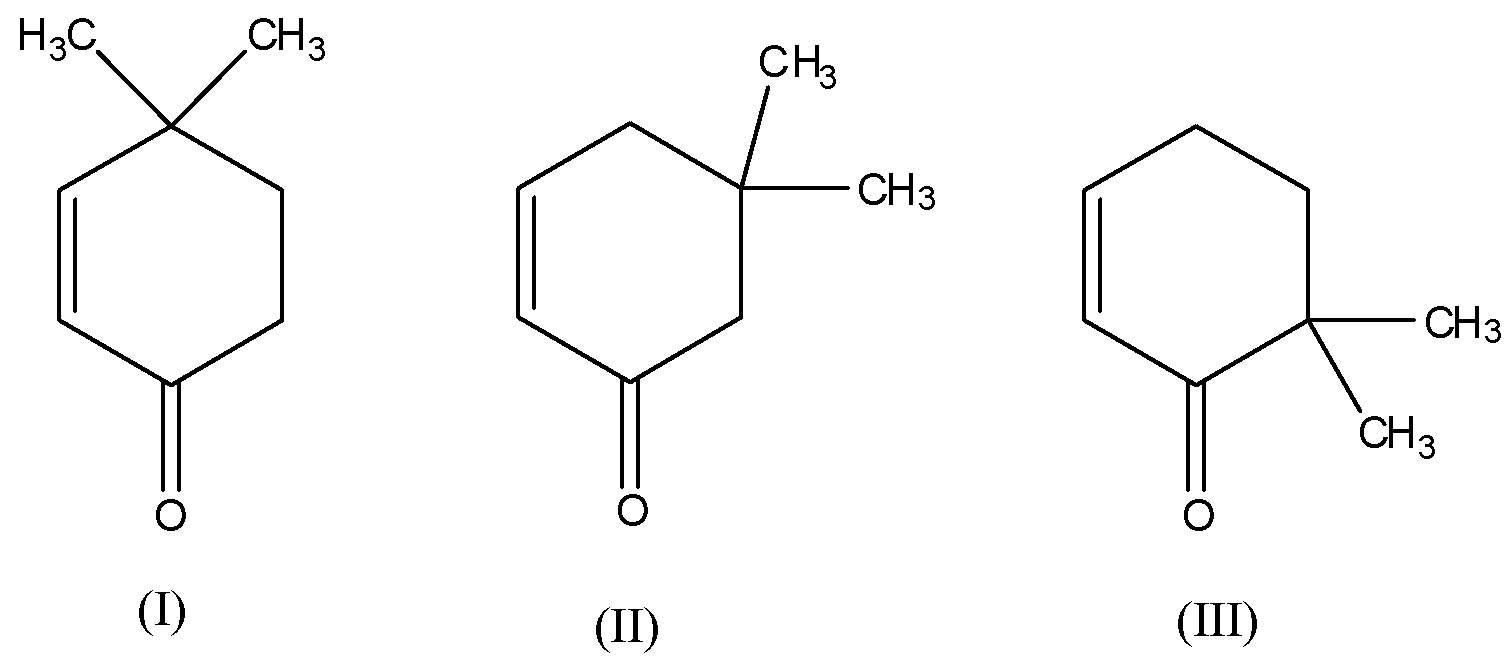
If you observe, all the three compounds are keto compounds or ketones and they have an acidic $\alpha - hydrogen$. Thus, they all will show tautomerism as shown below:


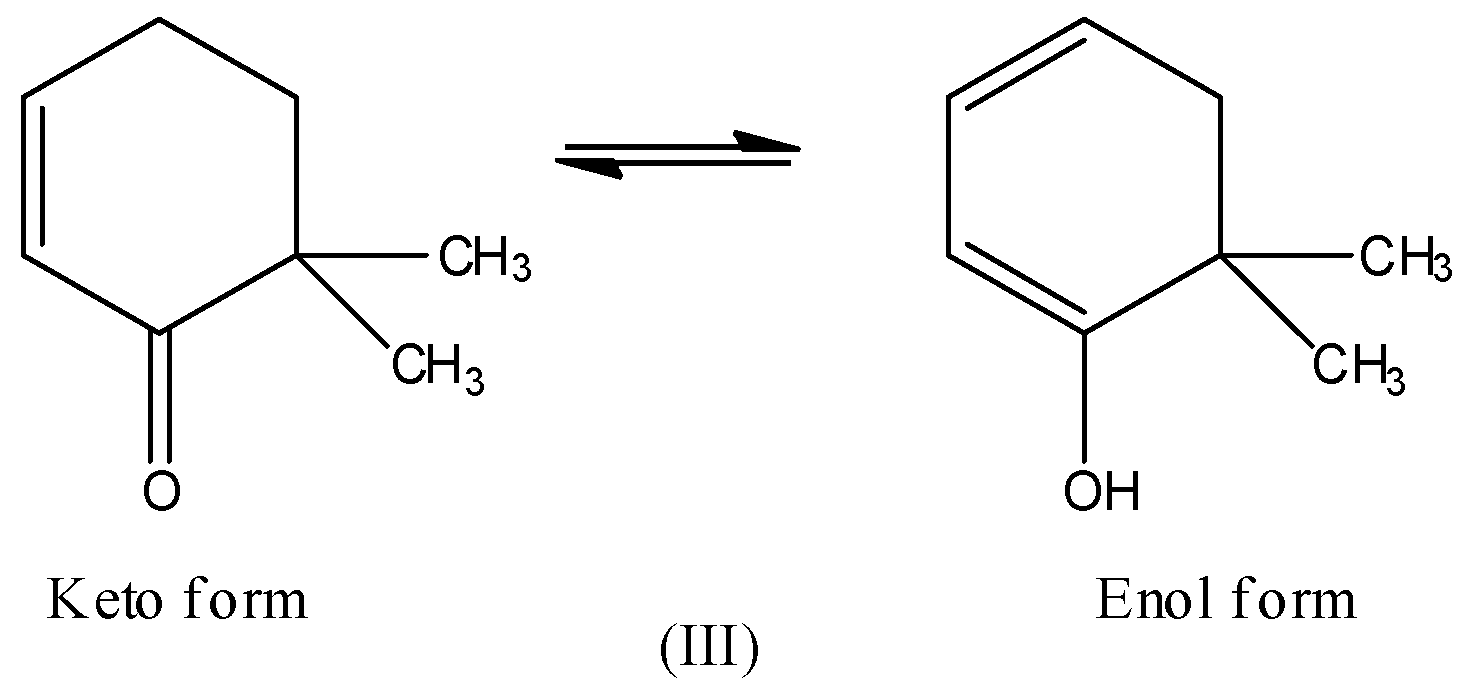
Hence, the correct option is B.
Note:
In keto-enol tautomerism, keto form is usually more stable than the enol form. This can be attributed to the fact that a carbon-oxygen double bond is more stronger than the carbon-carbon double bond. Thus, in most keto-enol tautomerism, the equilibrium lies more towards the keto form.
Complete answer:
Tautomerism is a functional isomerism wherein the two structures which are isomers readily interconvert to each other and exist in dynamic equilibrium with each other. This reaction results in the relocation of proton (hydrogen atom) and the two structural isomers are called tautomers. Now, the two conditions for tautomerism are:
1. Compounds must have an electron withdrawing atom or group i.e., atom which is more electronegative than carbon and has the tendency to accept the hydrogen atom (as tautomerism involves migration of hydrogen atom).
2. There must be a carbon linked to that electron withdrawing atom and further this carbon is linked to another carbon having a hydrogen atom. This hydrogen is referred to as acidic $\alpha - hydrogen$.
Keto compounds fulfill these conditions and the keto-enol tautomeric pair is one of the most common tautomeric pairs. Keto-enol tautomerism can be generally represented as:

Now, the given compounds are:

If you observe, all the three compounds are keto compounds or ketones and they have an acidic $\alpha - hydrogen$. Thus, they all will show tautomerism as shown below:



Hence, the correct option is B.
Note:
In keto-enol tautomerism, keto form is usually more stable than the enol form. This can be attributed to the fact that a carbon-oxygen double bond is more stronger than the carbon-carbon double bond. Thus, in most keto-enol tautomerism, the equilibrium lies more towards the keto form.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




