
Which of the following complexes is used to be an anticancer agent?
(A) mer-$[Co{(N{H_3})_3}Cl]$
(B) cis-$[PtC{l_2}{(N{H_3})_2}]$
(C) cis-${K_2}[PtC{l_2}B{r_2}]$
(D) $N{a_2}CoC{l_4}$
Answer
586.5k+ views
Hint: The complex which is used as an anticancer agent involves a precious metal. This complex has two isomers and one isomer is very highly effective against cancer. The synthesis of this complex involves treatment with ammonia.
Complete answer:
Let’s know which complex is used to cure the cancer.
- Cisplatin is the complex used for the treatment of cancer. The chemical we use in the treatment of cancer is also called an anticancer agent.
- The chemical formula of the Cisplatin is cis-$[PtC{l_2}{(N{H_3})_2}]$. Its molecular structure can be given as
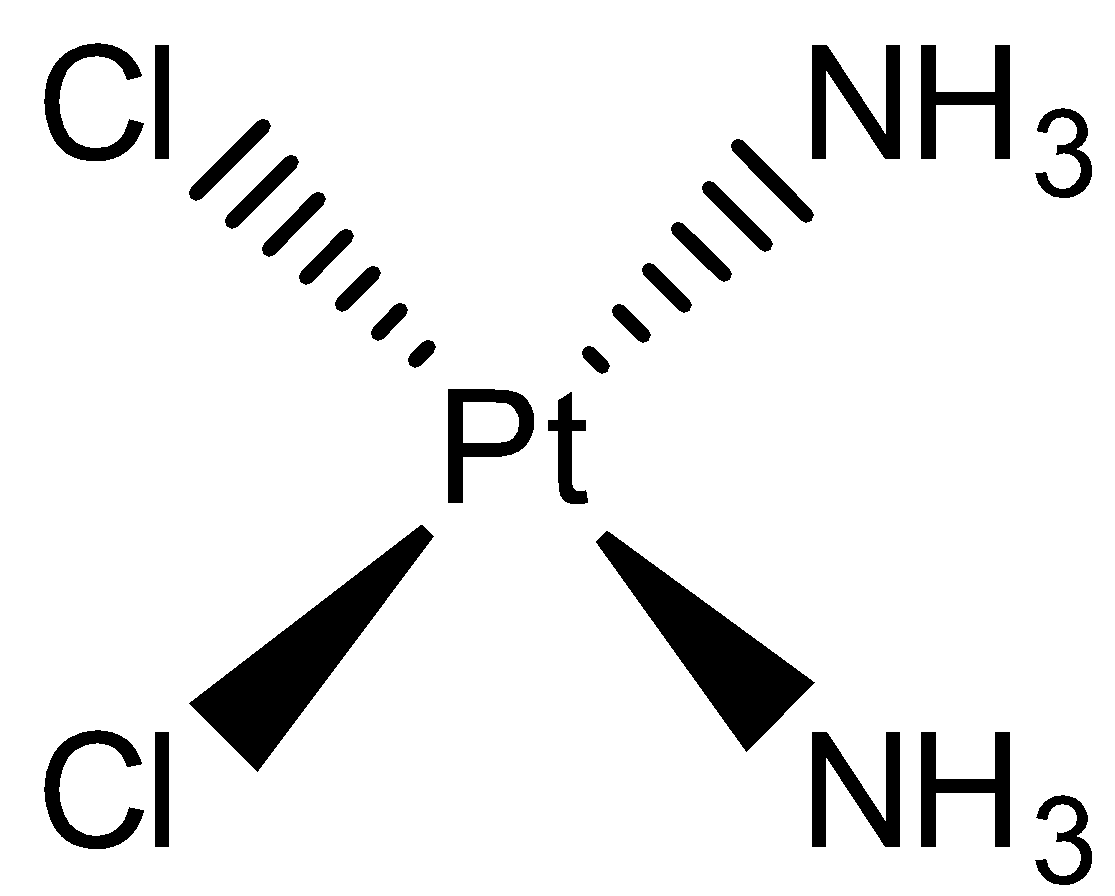
- We can see that this complex is a square planar complex. The oxidation number of Pt metal in this complex is +2.
- It can be obtained from potassium tetrachloroplatinate by allowing it to react with excess KI followed by ammonia. Then the product’s treatment with silver nitrate and exess KI gives cis-$[PtC{l_2}{(N{H_3})_2}]$ .
- With this anticancer agent we can treat many types of cancers including testicular cancer, breast cancer, ovarian cancer, lung cancer, etc.
- It is given intravenously into our body. That means that it is given by injection into our body.
- Is special use is in testicular cancer. When this drug was not discovered, the cure rate of the patients was 10% but after its discovery, the cure rate rose to 85%.
Thus, we can say that the correct answer is (B).
Note:
Cisplatin interferes with DNA replication and thus it kills the fastest proliferating cells. Generally those cells are cancerous. The trans isomer of this complex does not exhibit required pharmacological effect.
Complete answer:
Let’s know which complex is used to cure the cancer.
- Cisplatin is the complex used for the treatment of cancer. The chemical we use in the treatment of cancer is also called an anticancer agent.
- The chemical formula of the Cisplatin is cis-$[PtC{l_2}{(N{H_3})_2}]$. Its molecular structure can be given as

- We can see that this complex is a square planar complex. The oxidation number of Pt metal in this complex is +2.
- It can be obtained from potassium tetrachloroplatinate by allowing it to react with excess KI followed by ammonia. Then the product’s treatment with silver nitrate and exess KI gives cis-$[PtC{l_2}{(N{H_3})_2}]$ .
- With this anticancer agent we can treat many types of cancers including testicular cancer, breast cancer, ovarian cancer, lung cancer, etc.
- It is given intravenously into our body. That means that it is given by injection into our body.
- Is special use is in testicular cancer. When this drug was not discovered, the cure rate of the patients was 10% but after its discovery, the cure rate rose to 85%.
Thus, we can say that the correct answer is (B).
Note:
Cisplatin interferes with DNA replication and thus it kills the fastest proliferating cells. Generally those cells are cancerous. The trans isomer of this complex does not exhibit required pharmacological effect.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




