
which of the amino groups in semicarbazide will react with carbonyl groups?
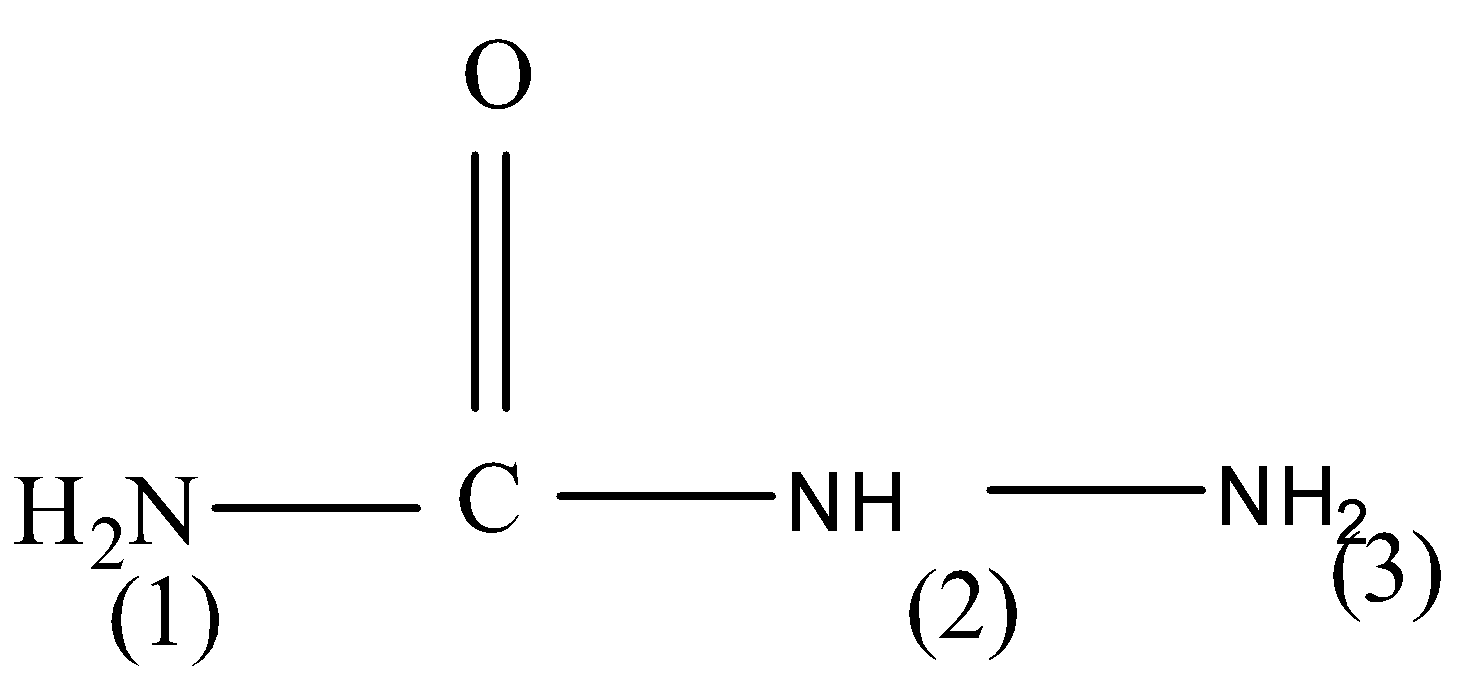
(A) $1$
(B) $2$
(C) $3$
(D) $1$ & $3$

Answer
559.5k+ views
Hint: The structure given in the equation is of semicarbazide and we can easily observe that there are two amino groups $\left( { - N{H_2}} \right) $ present in semicarbazide. As they are present in different electronic environments they will show different behavior while reacting with different groups. According to the question, we will predict which amino group in semicarbazide will react with the carbonyl group.
Complete step by step answer:
We will discuss all the three amine groups one by one which are numbered as $\left( 1 \right) ,\left( 2 \right) $ and $\left( 3 \right) $ . So let’s start one by one.
(1) In the first option we have given that $\left( 1 \right) $ amino group $\left( { - N{H_2}} \right) $ will react with the carbonyl group $\left( { - C = O} \right) $ . So, let’s study the environment of $\left( 1 \right) $ amino group $\left( { - N{H_2}} \right) $ . So, we can observe that in semicarbazide $\left( 1 \right) $ amino group $\left( { - N{H_2}} \right) $ is bonded with carbon only. In a highly acidic medium, the $\left( { - N{H_2}} \right) $ group gets protonated. Due to strong $\left( { - I} \right) $ effect of the protonated group, the lone pair of electrons on the $\left( { - N{H_2}} \right) $ group of protonated hydrazine is not available for the nucleophilic attack on the $\left( { - C = O} \right) $ group and hence hydrazine formation does not occur.
(2) The second $\left( { - NH} \right) $ group will also not be available for the nucleophilic attack on the $\left( { - C = O} \right) $ group and hence hydrazine formation does not occur.
(3) The third $\left( { - N{H_2}} \right) $ group which is the only reactive amine in semicarbazide. This $\left( { - N{H_2}} \right) $ group is attached to $\left( { - NH} \right) $ group has a lone pair of electrons and also available for nucleophilic attack on carbonyl group. Therefore, it will lead to formation of semicarbazones.
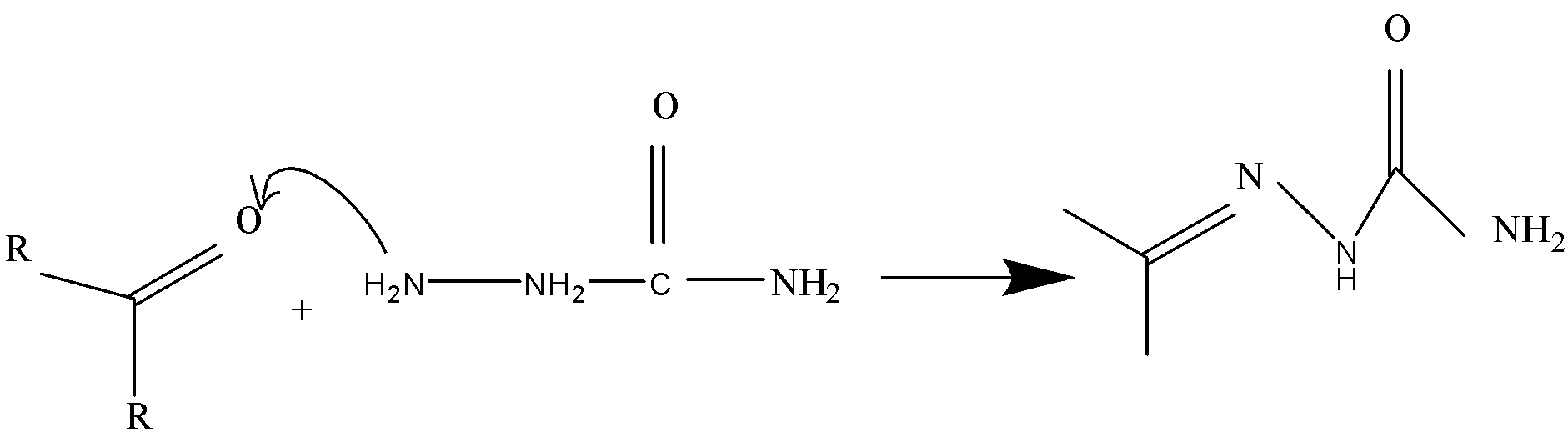
Final result: The correct option is, ‘(C) $3$’.
Note: The $\left( 1 \right) $ amino group $\left( { - N{H_2}} \right) $ is also called the amide functional group. It also has a lone pair on nitrogen atoms but the lone pair is involved in the resonance with the carbonyl group.
Complete step by step answer:
We will discuss all the three amine groups one by one which are numbered as $\left( 1 \right) ,\left( 2 \right) $ and $\left( 3 \right) $ . So let’s start one by one.
(1) In the first option we have given that $\left( 1 \right) $ amino group $\left( { - N{H_2}} \right) $ will react with the carbonyl group $\left( { - C = O} \right) $ . So, let’s study the environment of $\left( 1 \right) $ amino group $\left( { - N{H_2}} \right) $ . So, we can observe that in semicarbazide $\left( 1 \right) $ amino group $\left( { - N{H_2}} \right) $ is bonded with carbon only. In a highly acidic medium, the $\left( { - N{H_2}} \right) $ group gets protonated. Due to strong $\left( { - I} \right) $ effect of the protonated group, the lone pair of electrons on the $\left( { - N{H_2}} \right) $ group of protonated hydrazine is not available for the nucleophilic attack on the $\left( { - C = O} \right) $ group and hence hydrazine formation does not occur.
(2) The second $\left( { - NH} \right) $ group will also not be available for the nucleophilic attack on the $\left( { - C = O} \right) $ group and hence hydrazine formation does not occur.
(3) The third $\left( { - N{H_2}} \right) $ group which is the only reactive amine in semicarbazide. This $\left( { - N{H_2}} \right) $ group is attached to $\left( { - NH} \right) $ group has a lone pair of electrons and also available for nucleophilic attack on carbonyl group. Therefore, it will lead to formation of semicarbazones.

Final result: The correct option is, ‘(C) $3$’.
Note: The $\left( 1 \right) $ amino group $\left( { - N{H_2}} \right) $ is also called the amide functional group. It also has a lone pair on nitrogen atoms but the lone pair is involved in the resonance with the carbonyl group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




