
Which is more reactive, Benzaldehyde or propanal?
Answer
514.8k+ views
Hint: Let us first observe the structures of both benzaldehyde and propanal. Benzaldehyde has an aromatic group in the molecule whereas propanal only has an aliphatic carbon chain.
Benzaldehyde has the following structure:
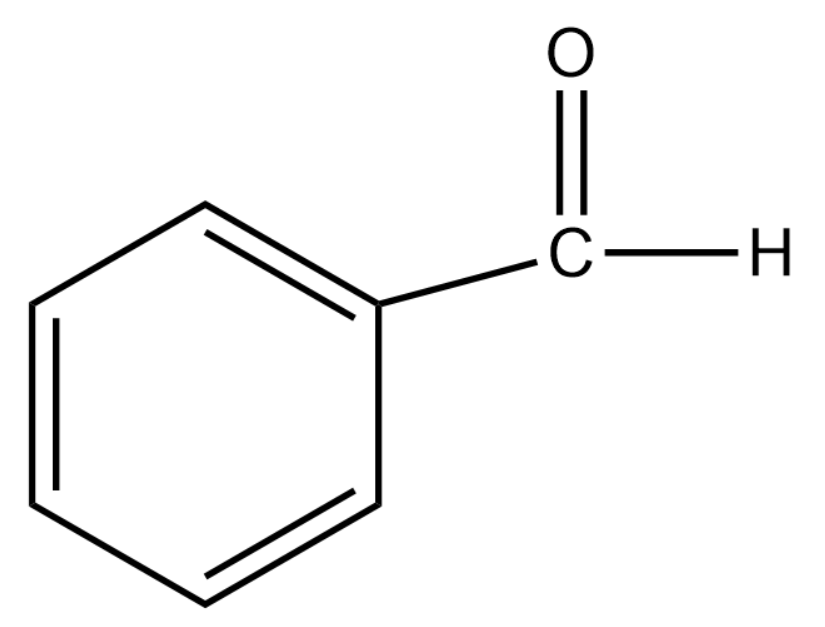
Propanal has the following structure:
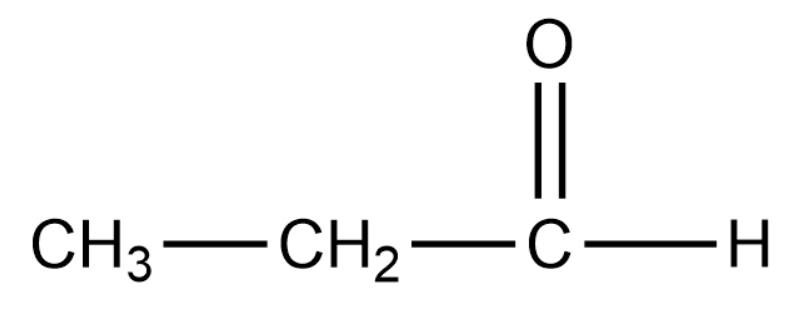
Complete answer:
The reactivity of Propanal is more than Benzaldehyde.
Reactivity of the compound that has an aldehyde group depends on the electrophilicity/the polarity of the carbonyl group. The carbonyl carbon should have partial positive charge and the oxygen should have a partial negative charge. Depending on the polarisation we can define the reactivity of the molecule. The better extent of polarisation means that the molecule is better reactive.
In the case of benzaldehyde, since the carbonyl carbon is attached to a benzene ring there is a reduction in the polarity. This is due to the fact that benzene undergoes resonance and the positive charge will be spread throughout the molecule. This makes it less reactive to nucleophilic reactions.
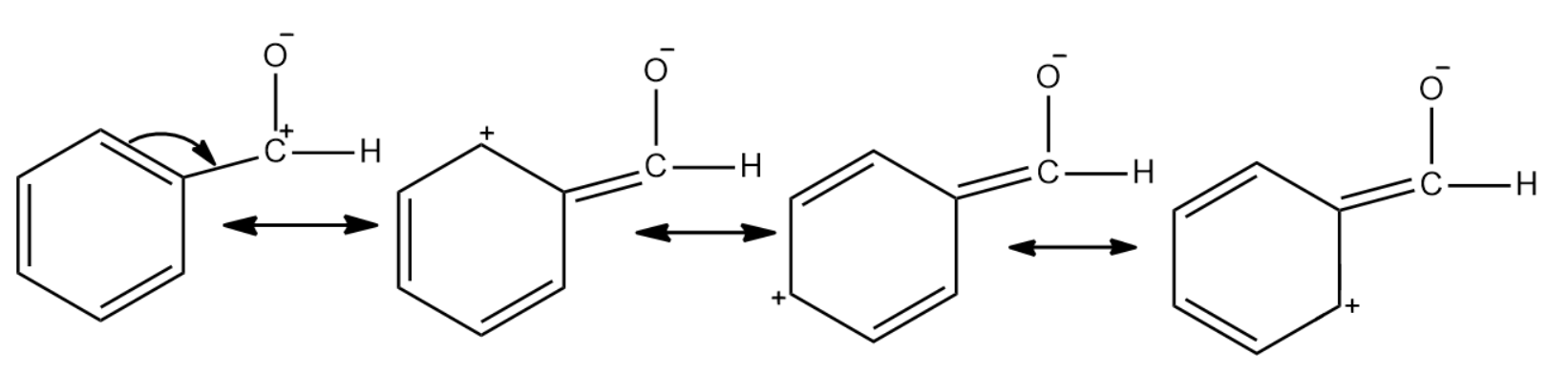
In the case of propanal there is no such resonance effect that comes into play. Due to this the polarity in the carbonyl group is usually unaffected. Thus the carbonyl carbon of benzaldehyde is less electrophilic than carbonyl carbon present in propanal.
This is the reason why Propanal is more reactive than Benzaldehyde.
Note:
Benzaldehyde is less reactive than propanal. This can also be interpreted as Benzaldehyde is more stable than propanal. All compounds that have an aromatic character are always more stable than the groups having aliphatic character. This is because resonance always stabilises a molecule.
Benzaldehyde has the following structure:

Propanal has the following structure:
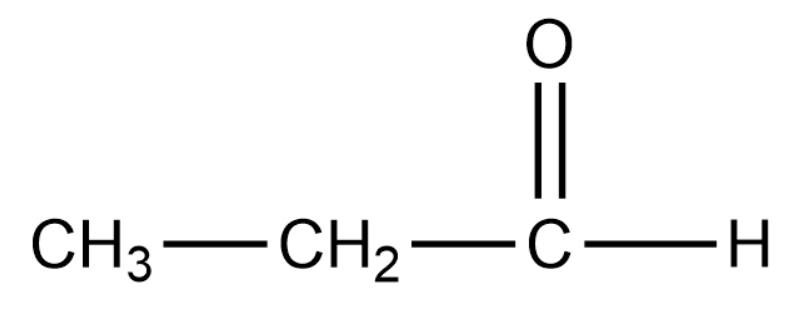
Complete answer:
The reactivity of Propanal is more than Benzaldehyde.
Reactivity of the compound that has an aldehyde group depends on the electrophilicity/the polarity of the carbonyl group. The carbonyl carbon should have partial positive charge and the oxygen should have a partial negative charge. Depending on the polarisation we can define the reactivity of the molecule. The better extent of polarisation means that the molecule is better reactive.
In the case of benzaldehyde, since the carbonyl carbon is attached to a benzene ring there is a reduction in the polarity. This is due to the fact that benzene undergoes resonance and the positive charge will be spread throughout the molecule. This makes it less reactive to nucleophilic reactions.

In the case of propanal there is no such resonance effect that comes into play. Due to this the polarity in the carbonyl group is usually unaffected. Thus the carbonyl carbon of benzaldehyde is less electrophilic than carbonyl carbon present in propanal.
This is the reason why Propanal is more reactive than Benzaldehyde.
Note:
Benzaldehyde is less reactive than propanal. This can also be interpreted as Benzaldehyde is more stable than propanal. All compounds that have an aromatic character are always more stable than the groups having aliphatic character. This is because resonance always stabilises a molecule.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




