
Which is an example of amorphous carbon?
A.Charcoal
B.Coke
C.Lamp black
D.All of the above
Answer
587.1k+ views
Hint: Basically, amorphous carbon is free, reactive carbon that does not have any crystalline structure. True amorphous carbon has localized pi-electrons and its bonds which are formed are inconsistent with other allotropes of carbon.
Complete step by step answer:
Generally, the phenomenon by which an element can exist in more than one physical state is called allotropy. There are two allotropes of carbon namely amorphous carbon allotropes and crystalline carbon allotropes. The allotropes of carbon can be either amorphous or crystalline.
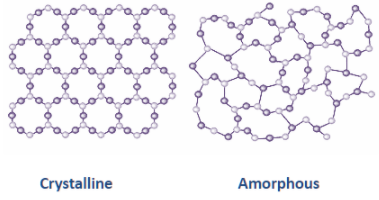
Now, in mineralogy, amorphous carbon is generally used for coal, soot, carbide-derived carbon and other impure forms of carbon that are neither graphite or diamond. Moreover, chemical bonds among atoms are a mixture of $s{p^2}$ and $s{p^3}$ hybridized bonds with a high concentration of bonds. Because the amorphous carbon is thermodynamically in a metastable state and the ratio of these bonds is variable, so the properties of amorphous carbon greatly depend on the formation methods and conditions. In the laboratory, amorphous carbon can be produced by physical vapor deposition, chemical vapor deposition and ion irradiation of diamond or graphite.
Now, all the options are correct as charcoal and coal are forms of amorphous carbon and lamp black is a type of carbon obtained from the soot of burned fat, oil, tar and resin.
Hence, option D is correct.
Note: Diamond is a well-known allotrope of carbon that exhibits hardness and high dispersion of light. The other one is graphene. It is a single layer of carbon atoms arranged in one plane. It is further a material of interest due to its high electron mobility and its application in electronics.
Complete step by step answer:
Generally, the phenomenon by which an element can exist in more than one physical state is called allotropy. There are two allotropes of carbon namely amorphous carbon allotropes and crystalline carbon allotropes. The allotropes of carbon can be either amorphous or crystalline.

Now, in mineralogy, amorphous carbon is generally used for coal, soot, carbide-derived carbon and other impure forms of carbon that are neither graphite or diamond. Moreover, chemical bonds among atoms are a mixture of $s{p^2}$ and $s{p^3}$ hybridized bonds with a high concentration of bonds. Because the amorphous carbon is thermodynamically in a metastable state and the ratio of these bonds is variable, so the properties of amorphous carbon greatly depend on the formation methods and conditions. In the laboratory, amorphous carbon can be produced by physical vapor deposition, chemical vapor deposition and ion irradiation of diamond or graphite.
Now, all the options are correct as charcoal and coal are forms of amorphous carbon and lamp black is a type of carbon obtained from the soot of burned fat, oil, tar and resin.
Hence, option D is correct.
Note: Diamond is a well-known allotrope of carbon that exhibits hardness and high dispersion of light. The other one is graphene. It is a single layer of carbon atoms arranged in one plane. It is further a material of interest due to its high electron mobility and its application in electronics.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




