
Which is an alicyclic compound?
A.Benzene
B.Cyclohexane
C.Pyridine
D.Pyrrole
Answer
595.2k+ views
Hint:
Alicyclic compounds are those compounds which are both aliphatic and cyclic and to not possess aromatic character. The Huckel rule is used to identify the cyclic and acyclic compound.
Complete step by step answer:
To identify the alicyclic compound from the given option, first we have to draw their structures and have to check if they are aromatic or not. If a compound is cyclic and aliphatic and non-aromatic, we can say that the compound is an alicyclic compound.
Let’s find the correct option.
-Option A is benzene. Now, we draw the structure of benzene.
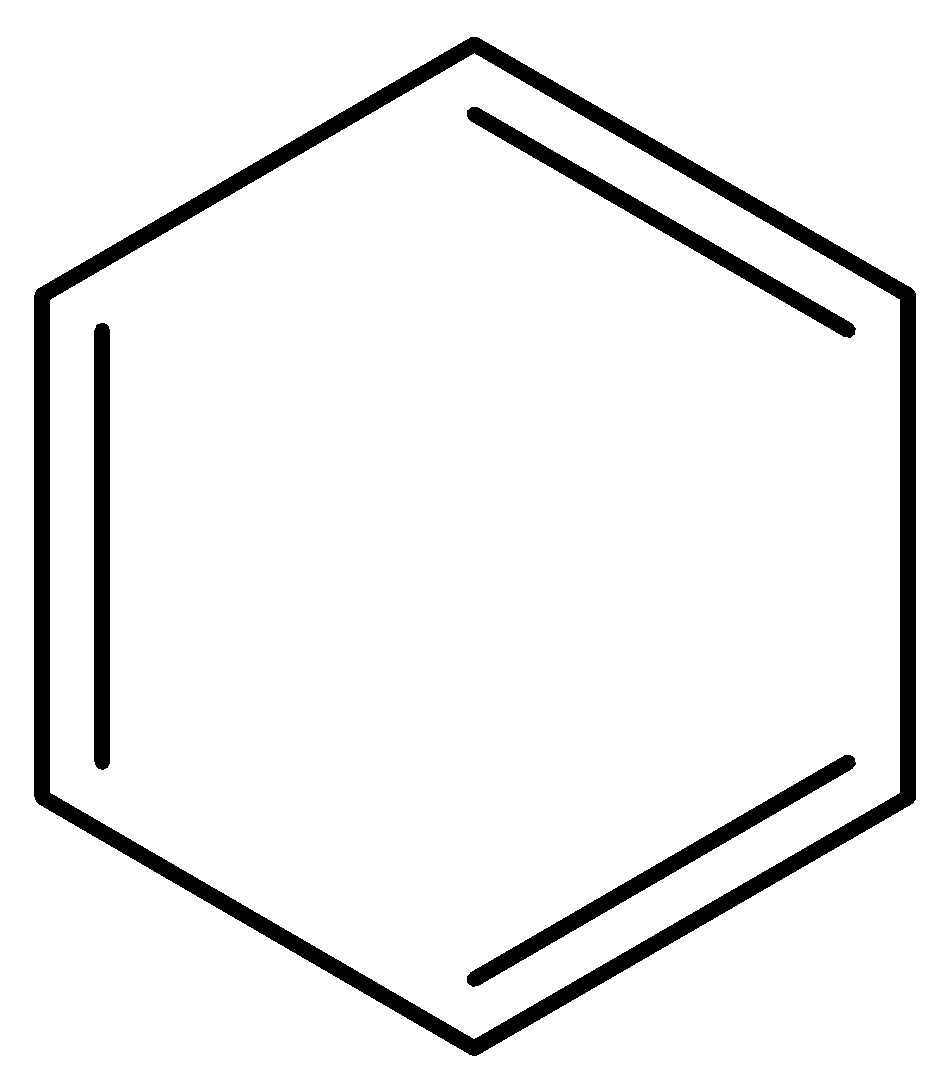
Benzene has alternate single and double bonds which causes conjugation in the compound. Also the compound obeys Huckel’s rule of (4n+2) pi electrons. So, benzene is an aromatic compound. But to be an alicyclic compound, a compound must be non-aromatic. So, option A is incorrect.
-Option B is cyclohexane. Now, we draw the structure of cyclohexane.
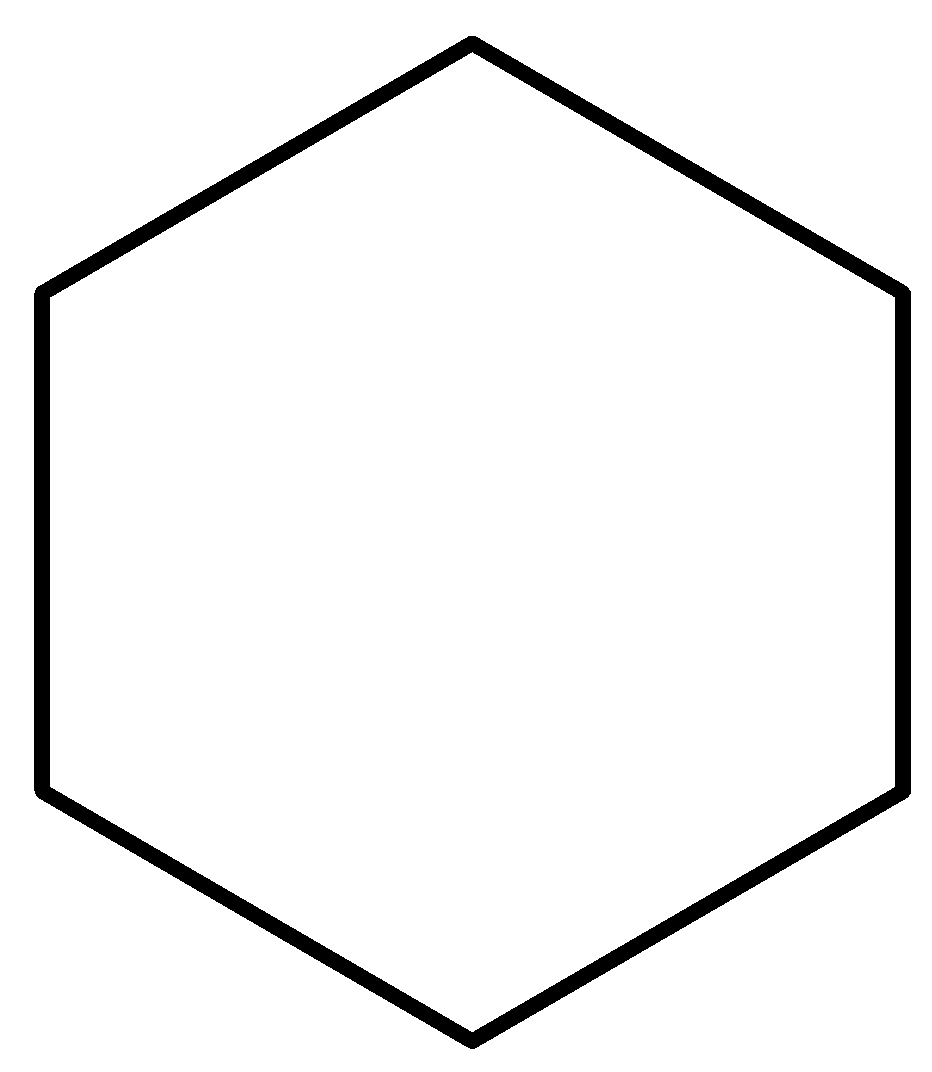
Cyclohexane is a cyclic saturated molecule. As no pi electrons are present, the compound is not aromatic. To be an alicyclic compound, a compound must be cyclic, aliphatic or non-aromatic. So, cyclohexane is an alicyclic compound.
-Option C is Pyridine. Now, we draw the structure of pyridine.
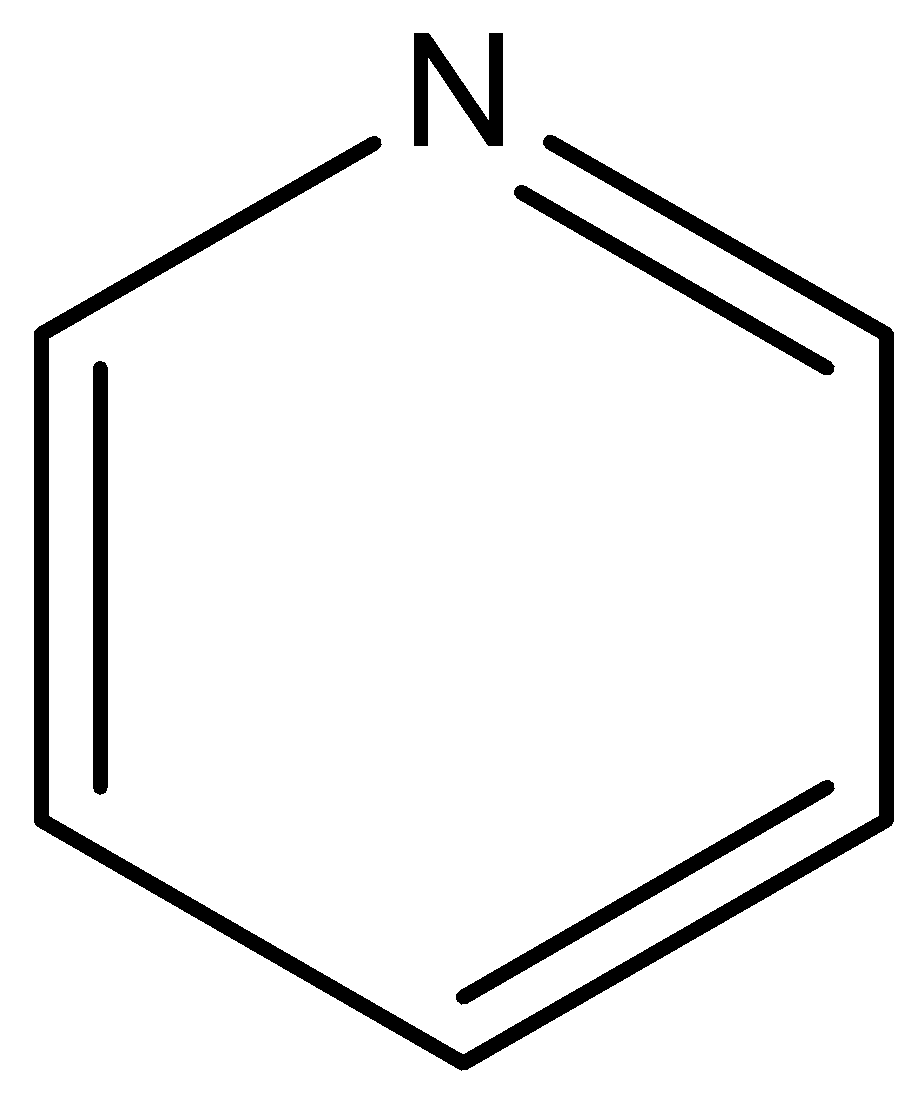
Pyridine is a cyclic molecule containing conjugated double bonds. Moreover, it follows Huckel's rule of 4n+2 pi electrons. So, it is an aromatic compound. Hence, option C is incorrect.
-Option D is pyrrole. Now, we draw the structure of Pyrrole.
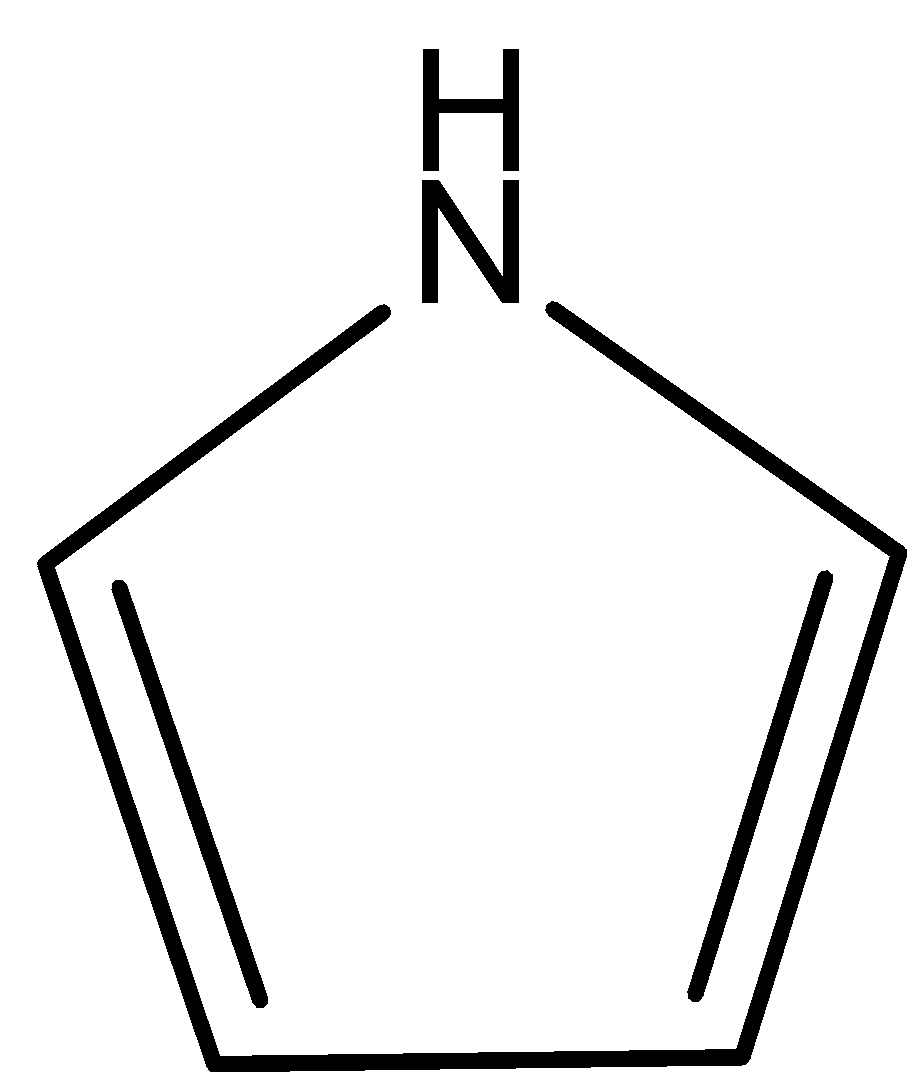
Pyrrole is a cyclic molecule possessing conjugated system. One lone pair and two pairs of pi bonds, that is, a total of six electrons contributed to the pi system. So, pyrrole follows the Hickel’s rule of 4n+2 pi electrons. So, it is an aromatic compound. So, option D is incorrect.
So, we concluded that option B is correct, that is, cyclohexane.
Note:
Huckel’ s rule of aromaticity states that if a cyclic and planar molecule has 4n+2 pi electrons, then the compound is said to be aromatic. Here, n indicates integer from 0 to n. If a compound possesses aromatic character, then it is not alicyclic.
Alicyclic compounds are those compounds which are both aliphatic and cyclic and to not possess aromatic character. The Huckel rule is used to identify the cyclic and acyclic compound.
Complete step by step answer:
To identify the alicyclic compound from the given option, first we have to draw their structures and have to check if they are aromatic or not. If a compound is cyclic and aliphatic and non-aromatic, we can say that the compound is an alicyclic compound.
Let’s find the correct option.
-Option A is benzene. Now, we draw the structure of benzene.

Benzene has alternate single and double bonds which causes conjugation in the compound. Also the compound obeys Huckel’s rule of (4n+2) pi electrons. So, benzene is an aromatic compound. But to be an alicyclic compound, a compound must be non-aromatic. So, option A is incorrect.
-Option B is cyclohexane. Now, we draw the structure of cyclohexane.

Cyclohexane is a cyclic saturated molecule. As no pi electrons are present, the compound is not aromatic. To be an alicyclic compound, a compound must be cyclic, aliphatic or non-aromatic. So, cyclohexane is an alicyclic compound.
-Option C is Pyridine. Now, we draw the structure of pyridine.

Pyridine is a cyclic molecule containing conjugated double bonds. Moreover, it follows Huckel's rule of 4n+2 pi electrons. So, it is an aromatic compound. Hence, option C is incorrect.
-Option D is pyrrole. Now, we draw the structure of Pyrrole.

Pyrrole is a cyclic molecule possessing conjugated system. One lone pair and two pairs of pi bonds, that is, a total of six electrons contributed to the pi system. So, pyrrole follows the Hickel’s rule of 4n+2 pi electrons. So, it is an aromatic compound. So, option D is incorrect.
So, we concluded that option B is correct, that is, cyclohexane.
Note:
Huckel’ s rule of aromaticity states that if a cyclic and planar molecule has 4n+2 pi electrons, then the compound is said to be aromatic. Here, n indicates integer from 0 to n. If a compound possesses aromatic character, then it is not alicyclic.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




