
Which has the maximum dipole moment?
A.
B.
C.
D.




Answer
558.9k+ views
Hint: We need to remember that in chemistry, dipole moment is observed whenever there is charge separation. In chemical compounds, dipole moment is found in ionic bonded compounds as well as in covalent bonded compounds also.
Complete step by step answer:
We know that whenever a bond is formed, it is either an ionic or a covalent bond. The atoms of different elements bond together to form a compound. Now the different elements may have different electronegativity. When two chemically bonded atoms have a good difference in electronegativity, a dipole moment is generated.
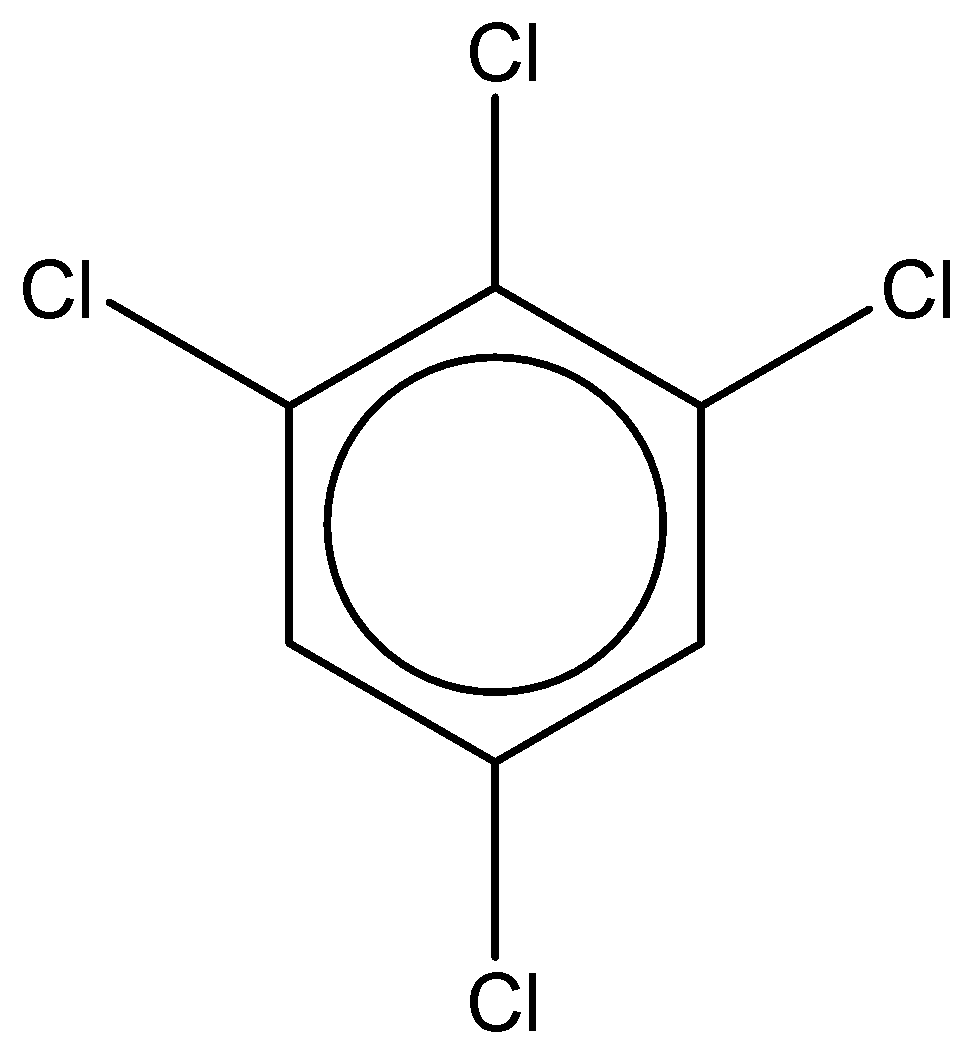
If we look at the first structure, it is 1,2,4,6-tetrachlorobenzene.
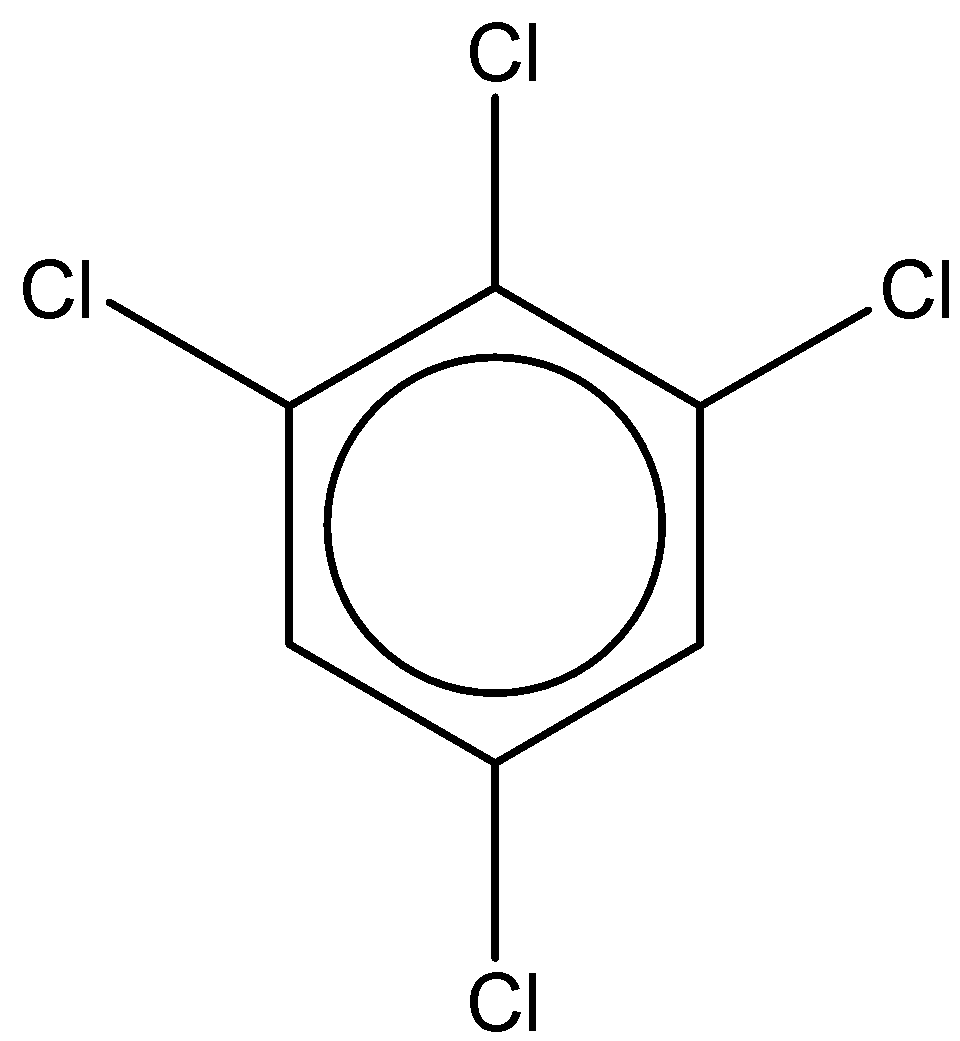
It has a chemical formula - ${C_6}{H_2}C{l_4}$
The dipole moment of this compound is found to be $0.65D$.
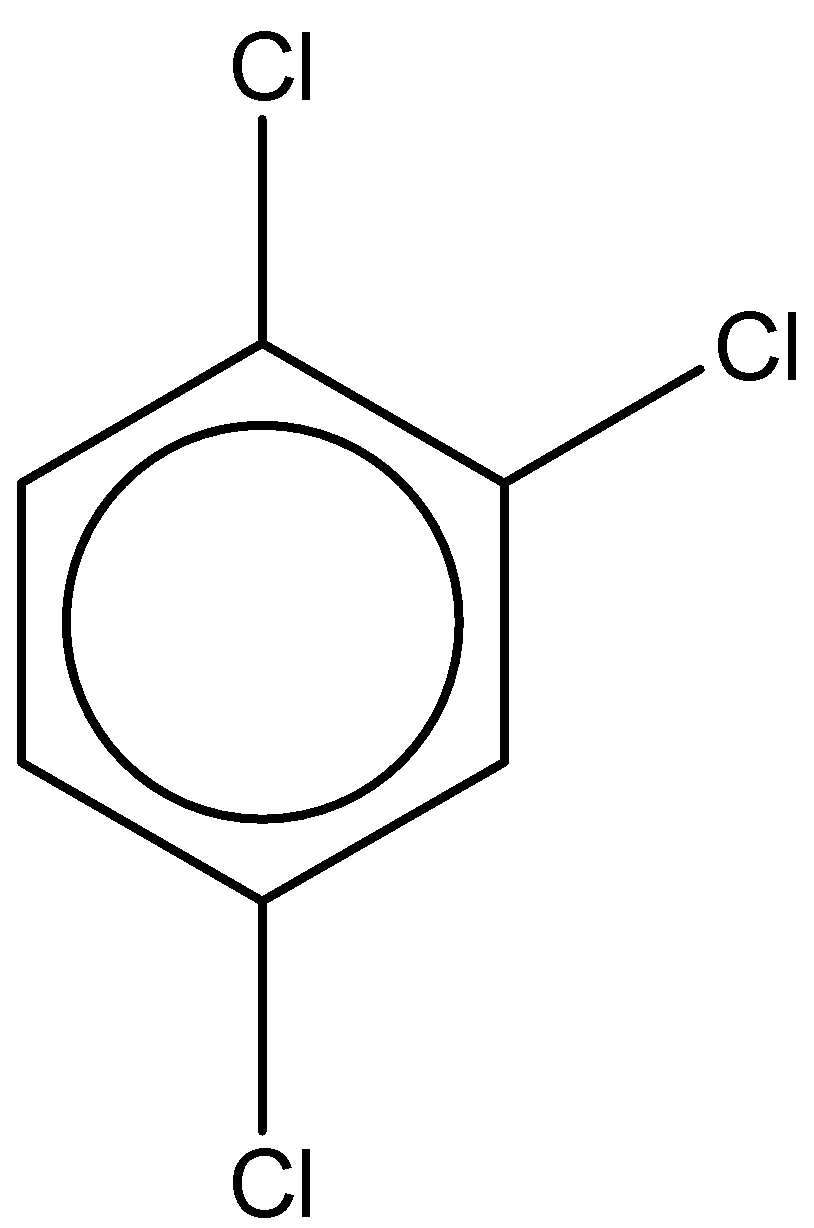
The second structure is 1,2,4-trichlorobenzene. It has a molecular formula – ${C_6}{H_3}C{l_3}$
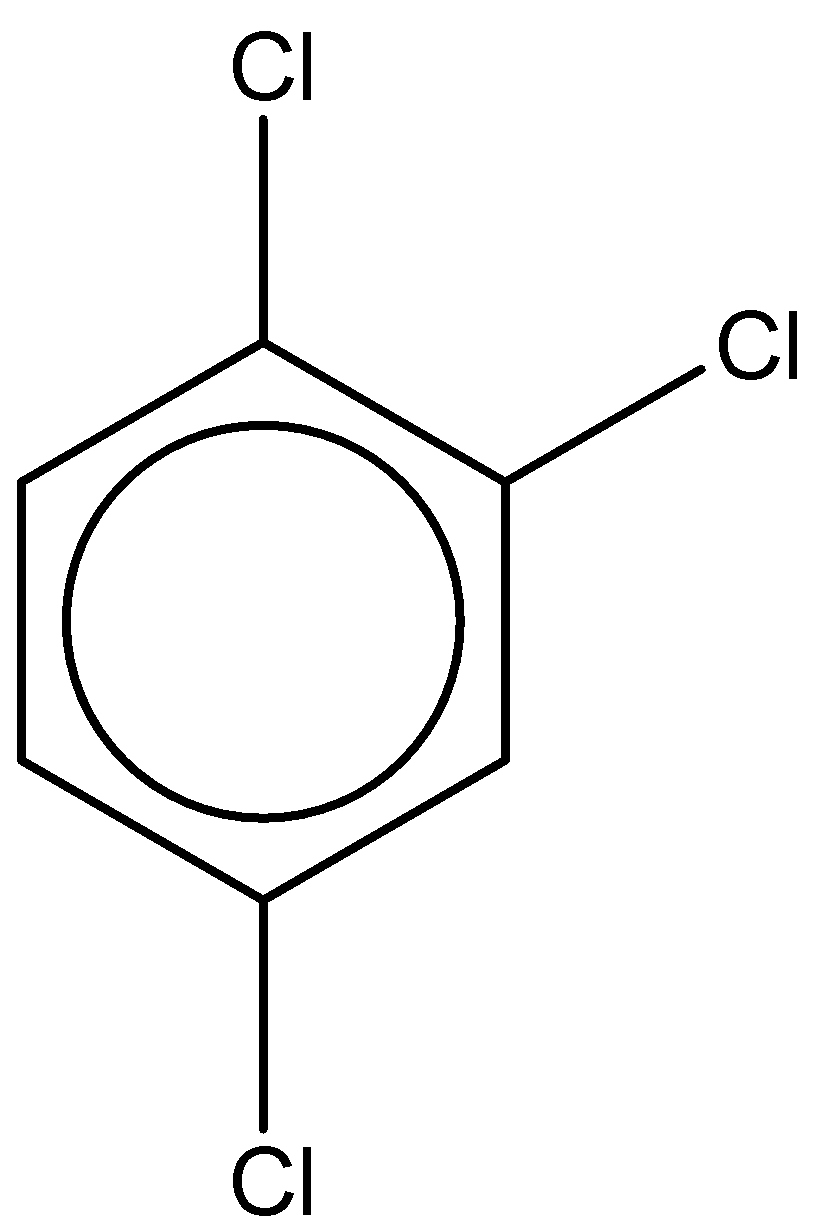
The dipole moment is found to be $1.26D$.
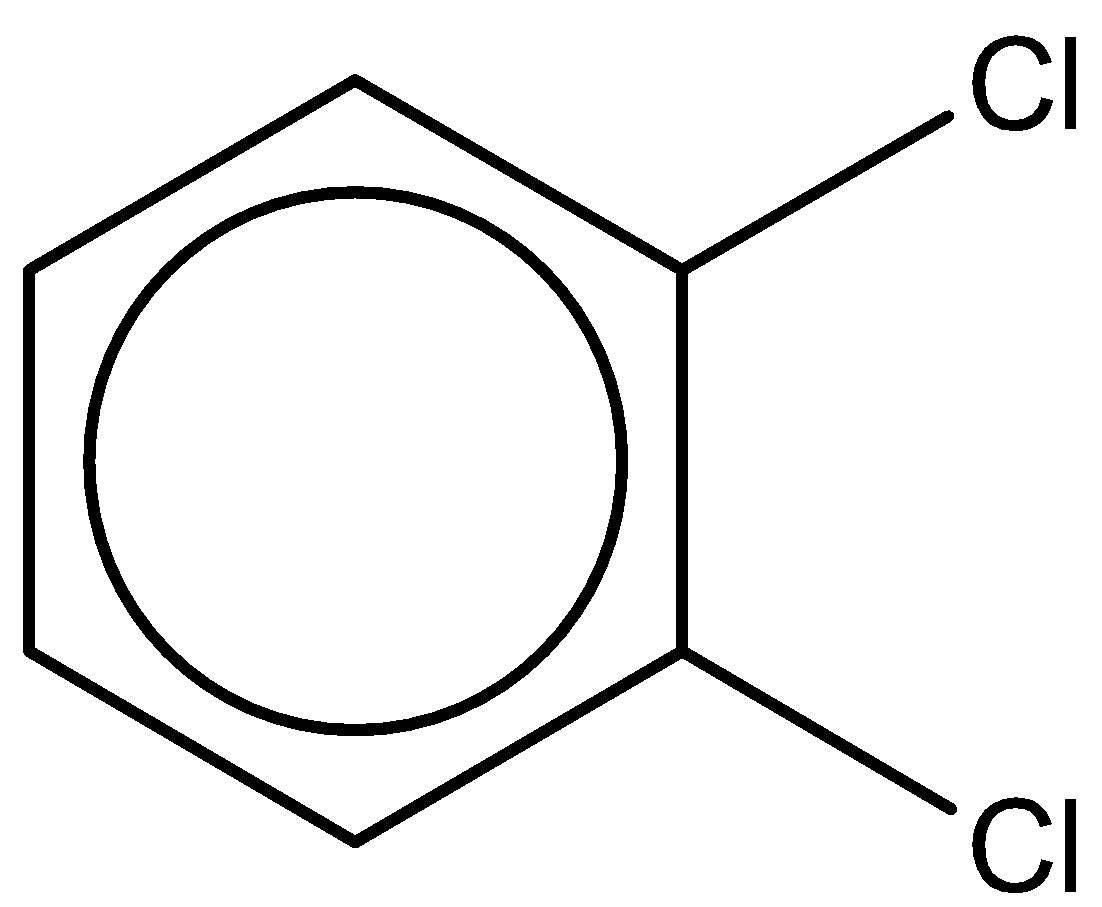
The third compound is 1,2-dichlorobenzene. It has a chemical formula - ${C_6}{H_4}C{l_2}$.
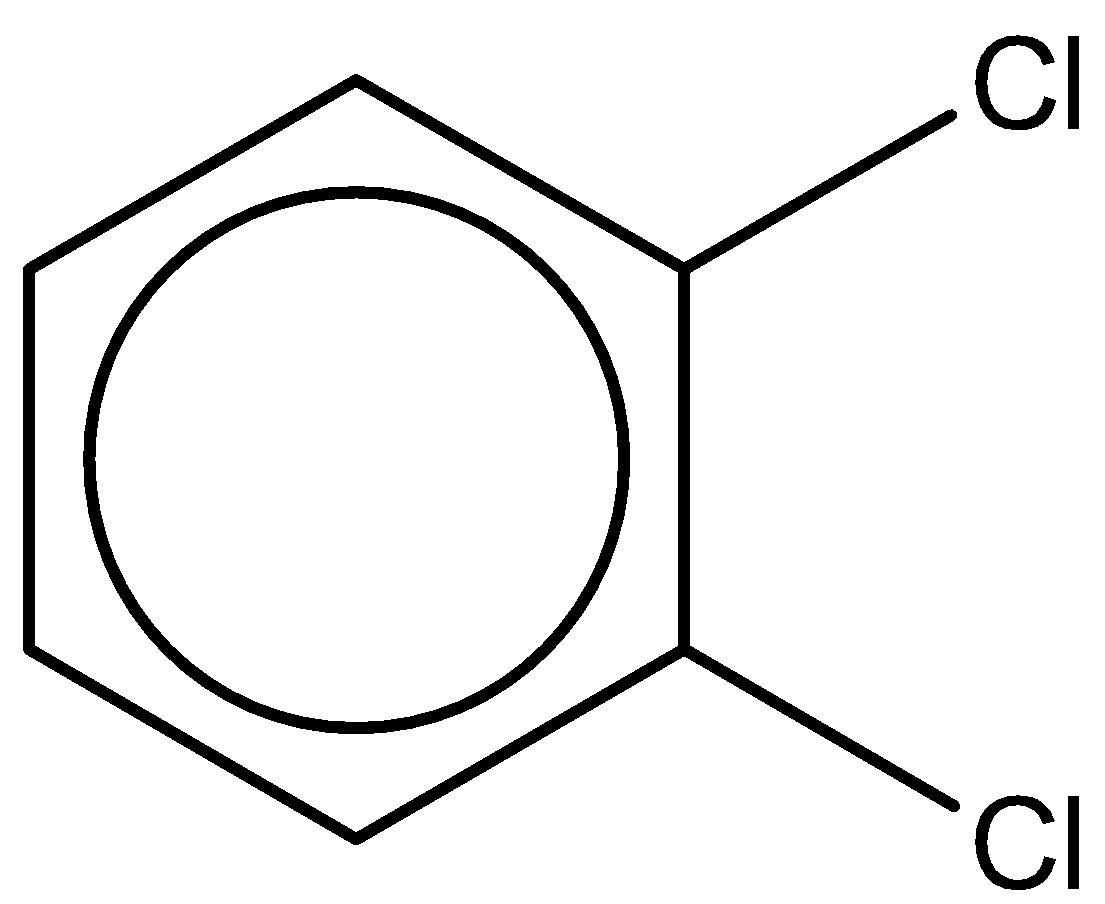
It has a dipole moment of $2.5D$.
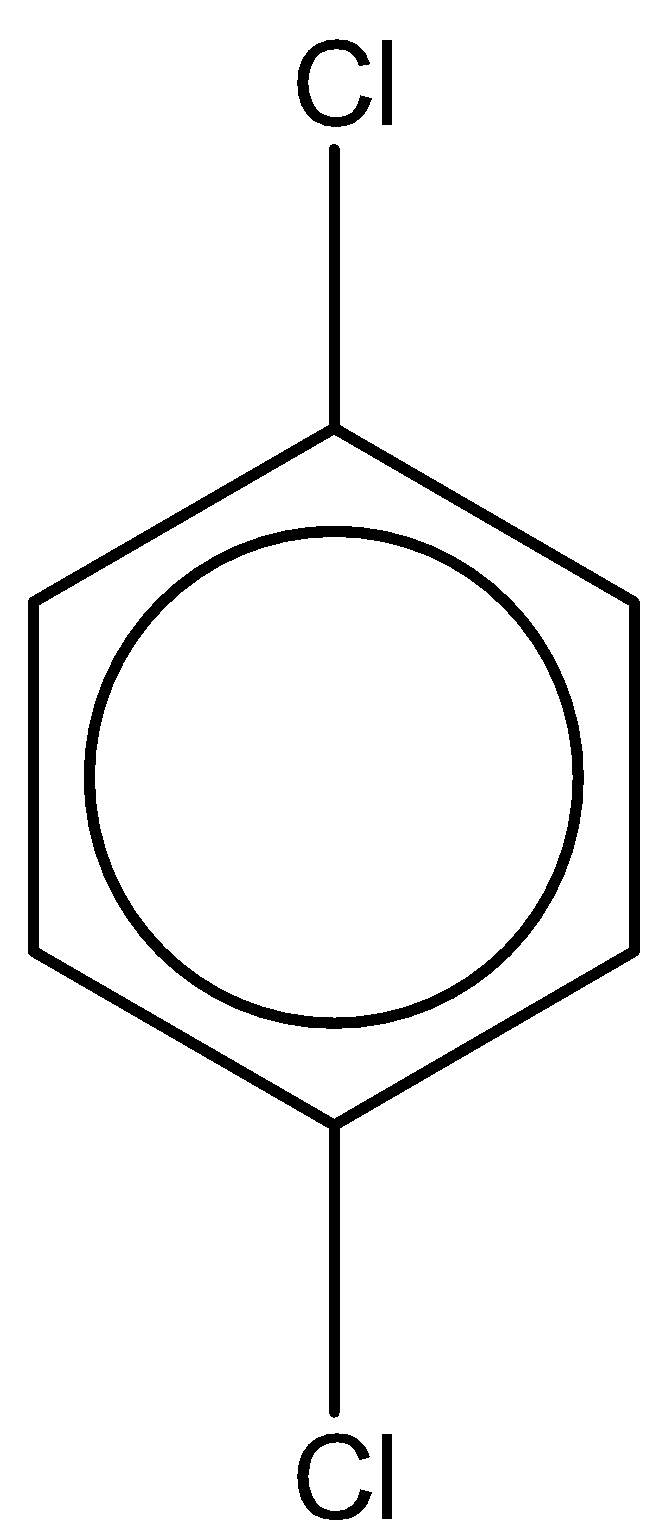
The last compound is 1,4-dichlorobenzene. With one chlorine atom at para and ortho position. The chemical formula is the same as 1,2-dichlorobenzene. But the dipole moment of this compound is 0 due to symmetry in the structure.
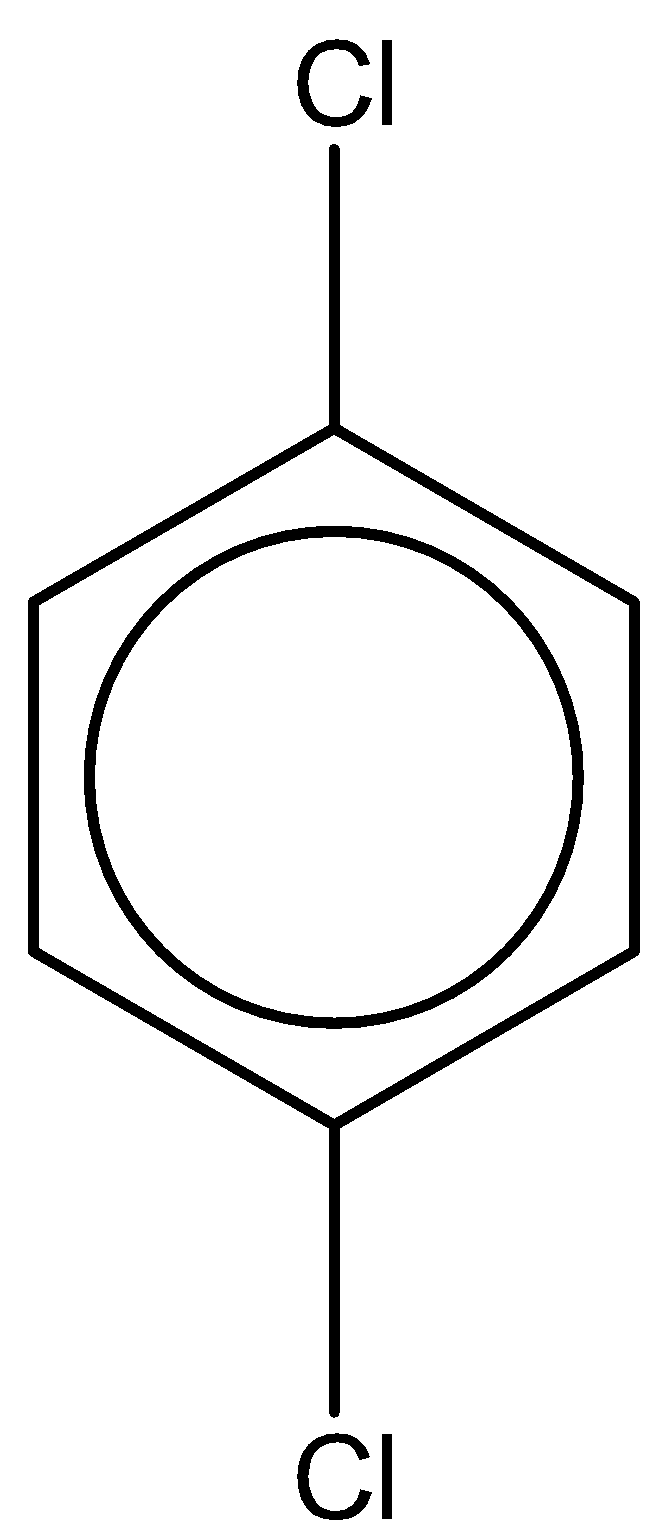
From this study, we can clearly observe that the compound 1,2-dichlorobenzene has the highest dipole moment of $2.5D$.
Thus, the correct answer to the question is option C.
Note: We should remember that in aromatic compounds the dipole moment can vary due to functional groups present at different positions. Same functional group present at ortho, para and meta positions may show different dipole moments and different properties. If there are any electron withdrawing groups like chlorine present in the benzene ring at different positions, then those at ortho will show more dipole moment then meta and para will have the least dipole moment.
Complete step by step answer:
We know that whenever a bond is formed, it is either an ionic or a covalent bond. The atoms of different elements bond together to form a compound. Now the different elements may have different electronegativity. When two chemically bonded atoms have a good difference in electronegativity, a dipole moment is generated.
If we look at the first structure, it is 1,2,4,6-tetrachlorobenzene.

It has a chemical formula - ${C_6}{H_2}C{l_4}$
The dipole moment of this compound is found to be $0.65D$.
The second structure is 1,2,4-trichlorobenzene. It has a molecular formula – ${C_6}{H_3}C{l_3}$

The dipole moment is found to be $1.26D$.
The third compound is 1,2-dichlorobenzene. It has a chemical formula - ${C_6}{H_4}C{l_2}$.

It has a dipole moment of $2.5D$.
The last compound is 1,4-dichlorobenzene. With one chlorine atom at para and ortho position. The chemical formula is the same as 1,2-dichlorobenzene. But the dipole moment of this compound is 0 due to symmetry in the structure.

From this study, we can clearly observe that the compound 1,2-dichlorobenzene has the highest dipole moment of $2.5D$.
Thus, the correct answer to the question is option C.
Note: We should remember that in aromatic compounds the dipole moment can vary due to functional groups present at different positions. Same functional group present at ortho, para and meta positions may show different dipole moments and different properties. If there are any electron withdrawing groups like chlorine present in the benzene ring at different positions, then those at ortho will show more dipole moment then meta and para will have the least dipole moment.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




