
Which has peroxy linkage?
(a) Perchloric acid
(b) Hypochlorous acid
(c) Para perchloric acid
(d) none of these
Answer
576.6k+ views
Hint:. Peroxy linkage is the linkage of the two oxygen atoms i.e. they are the oxygen-oxygen linkages and the compounds having such linkages are called the peroxy compounds. Now you can easily identify the compound with peroxy linkage.
Complete step by step answer:
First of all, let’s discuss what is peroxy linkage. By the peroxy linkage we mean that the compound consists of the -O-O- bond in it i.e. it has oxygen -oxygen linkage in it .
This peroxy linkage is very weak and results into the reactive free radicals when they undergo homolytic cleavage (the cleavage in which each atom receives one electron by the breaking of the covalent bond and results in the formation of the free radical is called s the homolytic cleavage).
Now considering the given options one by one as;
(a) Perchloric acid:- The formula of perchloric acid is $HCl{{O}_{4}}$ and its structure is as:
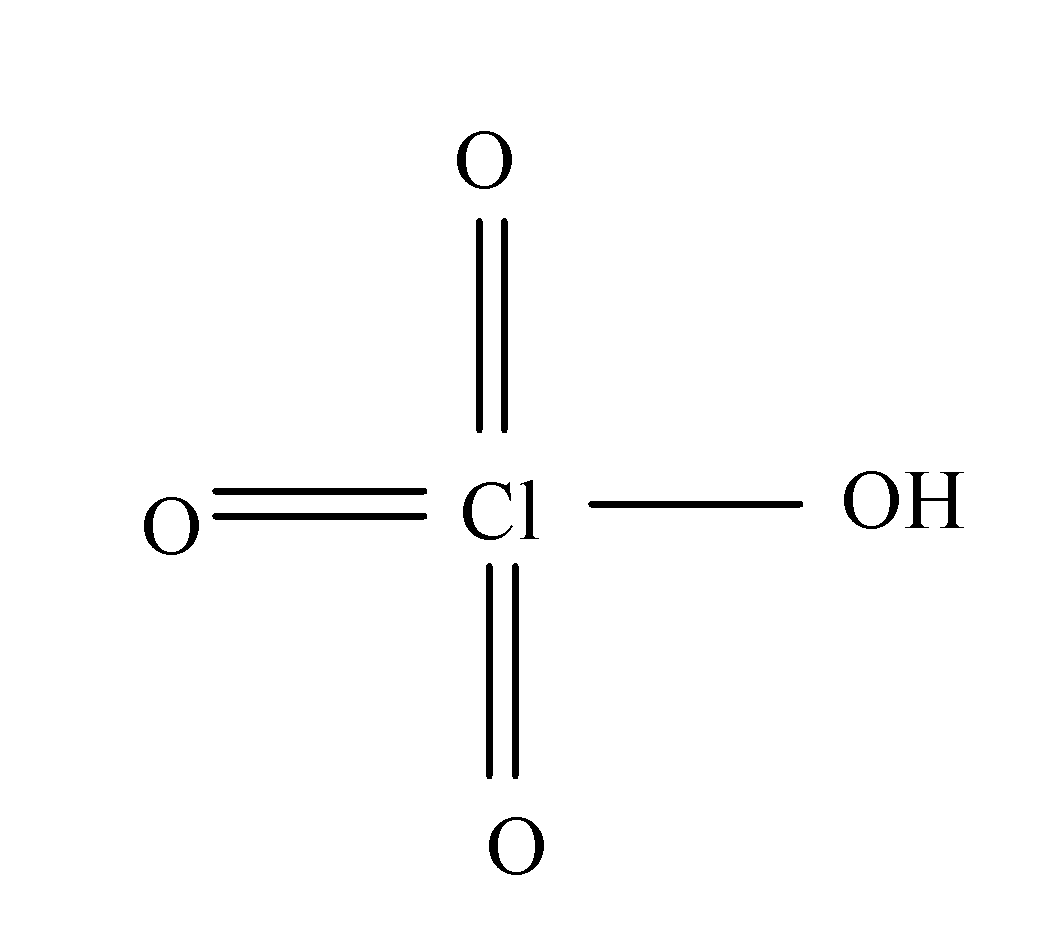
So, from its structure it is clear that it doesn’t consist of peroxy linkage.
(b) Hypochlorous acid:- The formula of hypochlorous acid is $H Cl O$ and its structure is as;
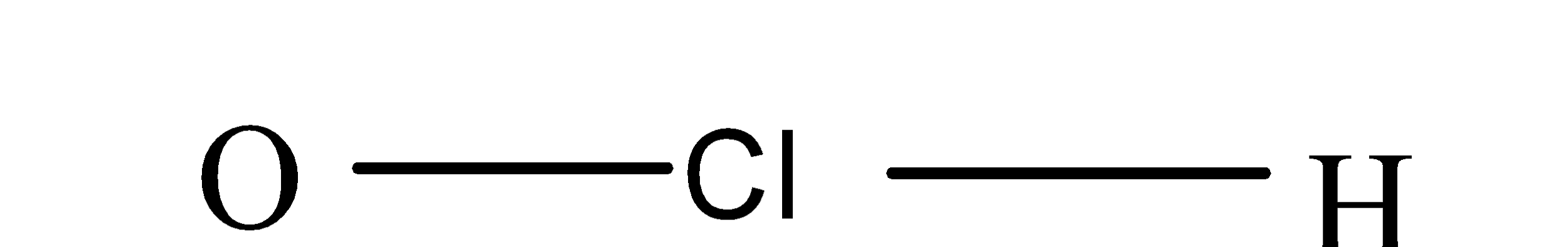
So, from its structure it is clear that it also doesn’t consist of peroxy linkage.
(c) Para perchloric acid:- The formula and structure of par perchloric acid is the same as that of the perchloric acid and has the same structure.
So, it also doesn’t consist of peroxy linkage.
Hence, neither of the above-mentioned compounds consists of the peroxy linkage.
So, the correct answer is “Option D”.
Note: Peroxy compounds are used in the process of polymerization and as well as in the addition reactions in the form of free radicals and they are also used as disinfectants, for bleaching purposes and in the manufacture of organic compounds like phenol through the cumene hyperoxide etc.
Complete step by step answer:
First of all, let’s discuss what is peroxy linkage. By the peroxy linkage we mean that the compound consists of the -O-O- bond in it i.e. it has oxygen -oxygen linkage in it .
This peroxy linkage is very weak and results into the reactive free radicals when they undergo homolytic cleavage (the cleavage in which each atom receives one electron by the breaking of the covalent bond and results in the formation of the free radical is called s the homolytic cleavage).
Now considering the given options one by one as;
(a) Perchloric acid:- The formula of perchloric acid is $HCl{{O}_{4}}$ and its structure is as:

So, from its structure it is clear that it doesn’t consist of peroxy linkage.
(b) Hypochlorous acid:- The formula of hypochlorous acid is $H Cl O$ and its structure is as;
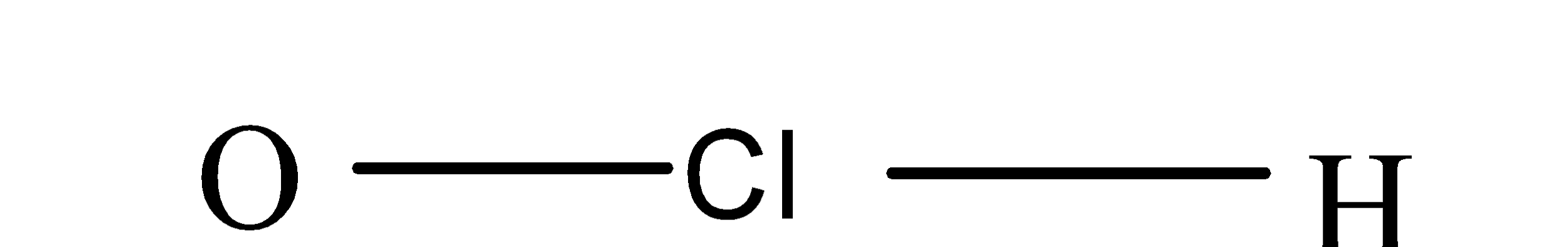
So, from its structure it is clear that it also doesn’t consist of peroxy linkage.
(c) Para perchloric acid:- The formula and structure of par perchloric acid is the same as that of the perchloric acid and has the same structure.
So, it also doesn’t consist of peroxy linkage.
Hence, neither of the above-mentioned compounds consists of the peroxy linkage.
So, the correct answer is “Option D”.
Note: Peroxy compounds are used in the process of polymerization and as well as in the addition reactions in the form of free radicals and they are also used as disinfectants, for bleaching purposes and in the manufacture of organic compounds like phenol through the cumene hyperoxide etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




