
Which has maximum heat of hydrogenation?
A)
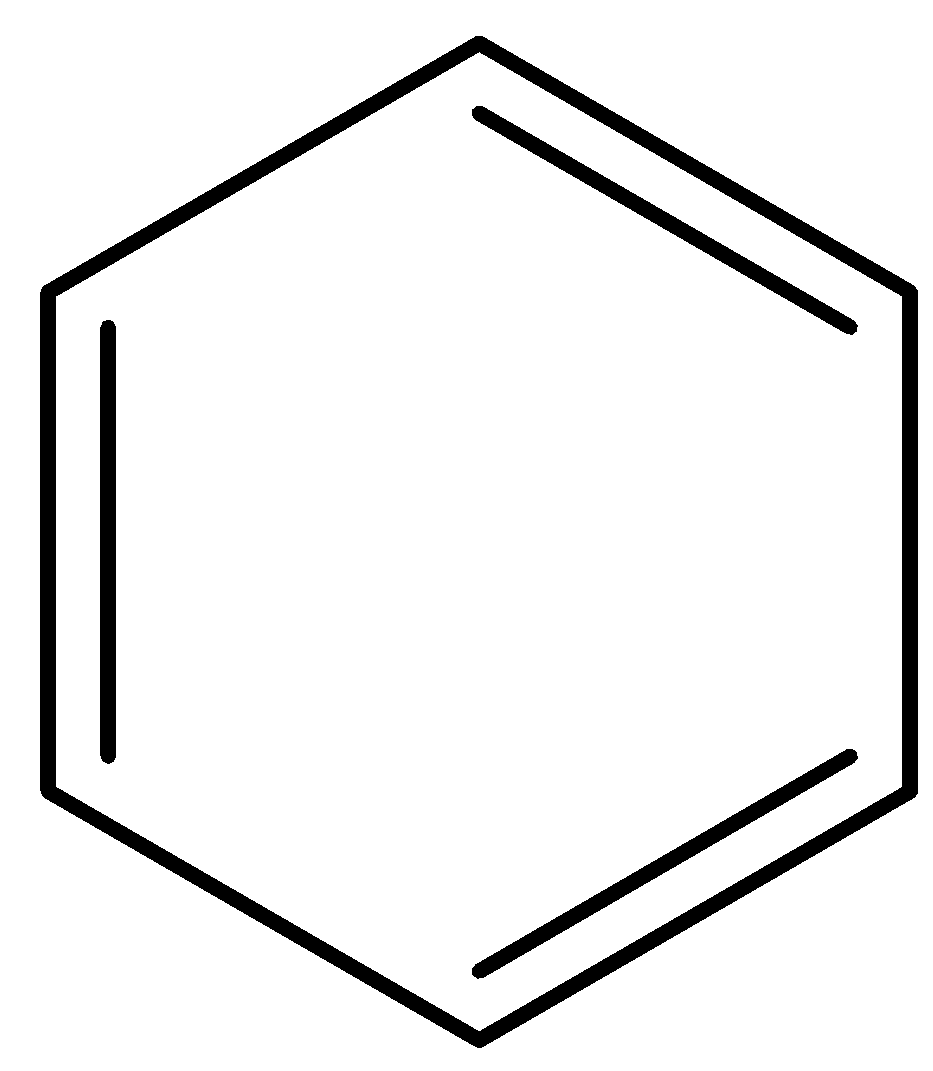
B)
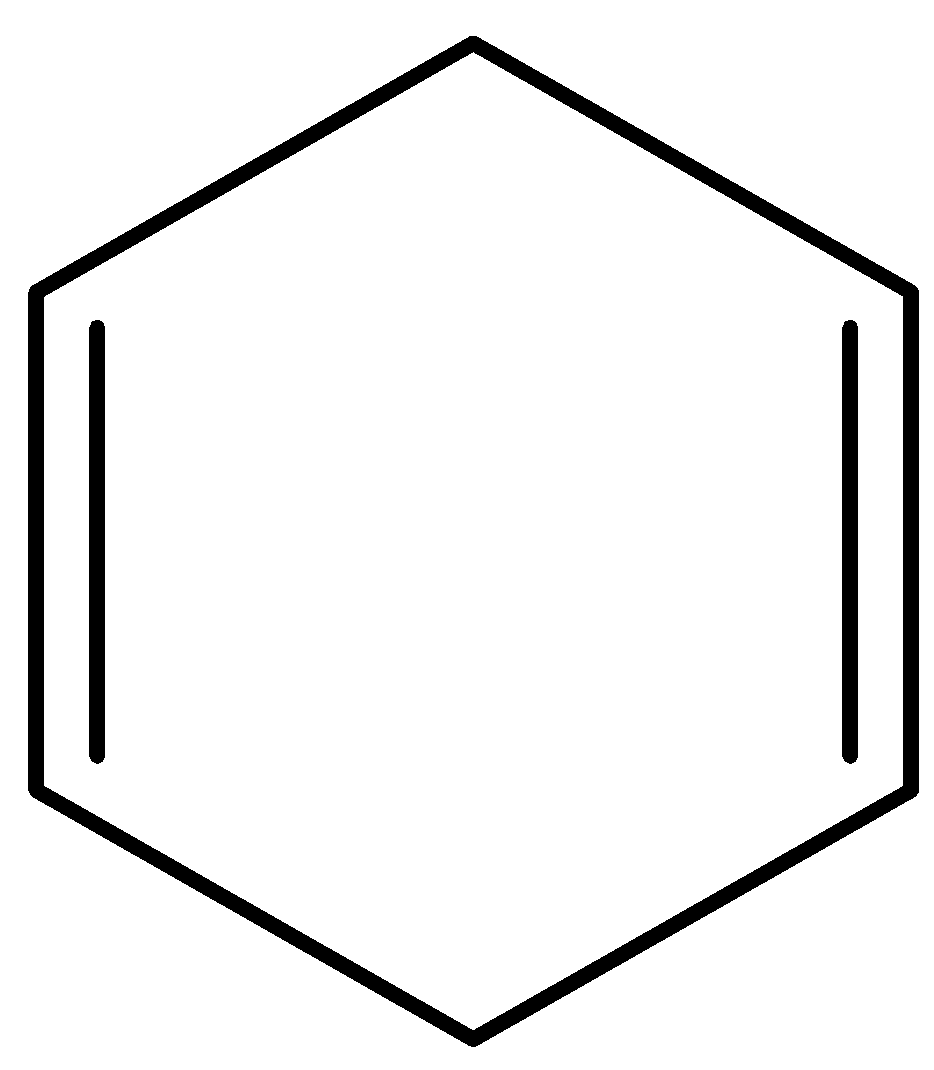
C)
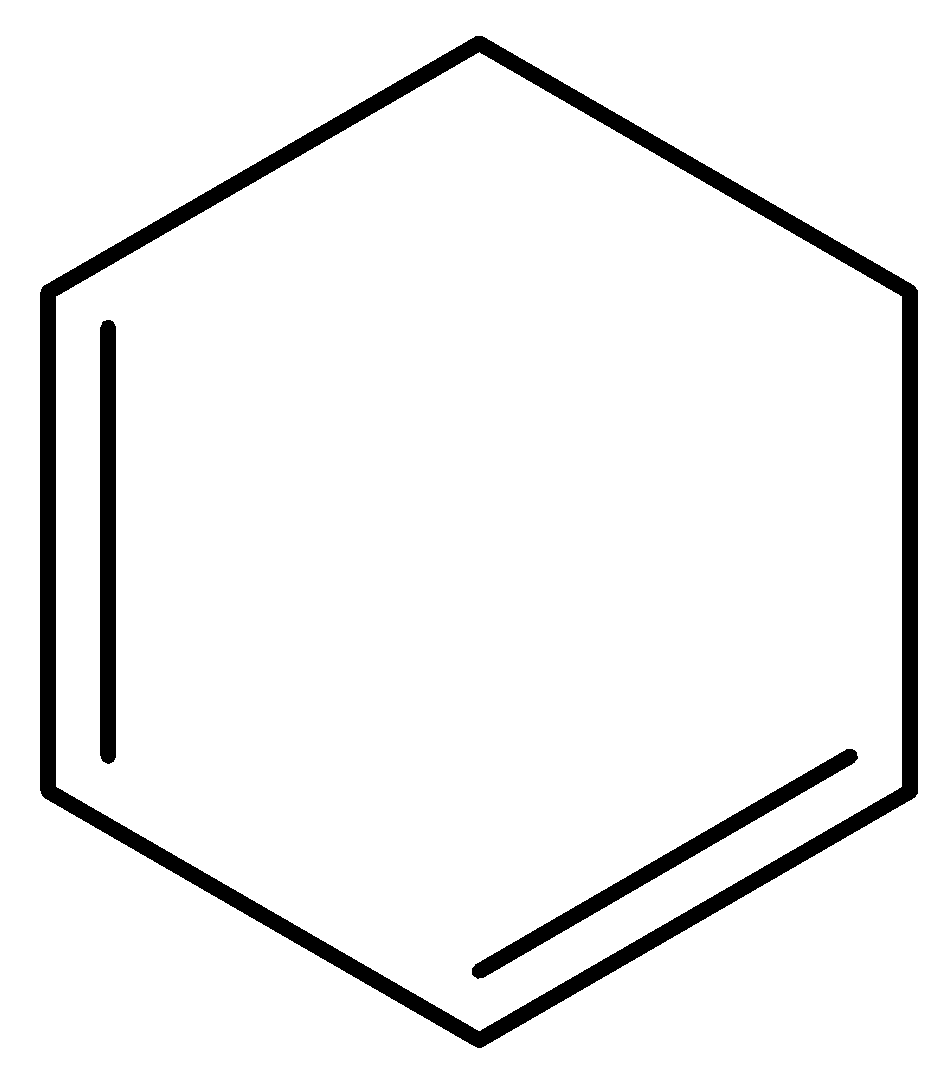
D) All have same values



Answer
519k+ views
Hint: We must have to know that the heat of hydrogenation is inversely proportional to the stability of a molecule. Benzene is highly stable in nature. Heat of hydrogenation is the measure of carbon double bond carbon. The smaller the value of heat of hydrogenation stronger is the double bond.
Complete answer:
We also remember that heat of hydrogenation is the stability of an alkene formed. In hydrogenation reactions, the bond is broken or formed; this defines the stability of an alkene and the heat of hydrogenation whether lower or higher.
Option A) this is an incorrect option as this structure is known as benzene and it is aromatic in nature and most stable among all the options given thus it has minimum value of heat of hydrogenation as the more stable the molecule less value for heat of hydrogenation.
Option B) This option is correct as this is 1,4 cyclohexadiene. It is a non-conjugated diene as the double bonds do not lie in conjugation thus it is least stable due to which maximum value for heat of hydrogenation.
Option C) this is an incorrect option.
Option D) this is an incorrect option as all the options will have different values for heat of hydrogenation depending upon the stability.
Note:
We have to remember that hydrogenation is nothing but chemical bond formation between hydrogen atoms and other molecules or compounds. The higher the double bond stability, minimum will be the value for heat of hydrogenation. Benzene is the most stable molecule with complete conjugation in it.
Complete answer:
We also remember that heat of hydrogenation is the stability of an alkene formed. In hydrogenation reactions, the bond is broken or formed; this defines the stability of an alkene and the heat of hydrogenation whether lower or higher.
Option A) this is an incorrect option as this structure is known as benzene and it is aromatic in nature and most stable among all the options given thus it has minimum value of heat of hydrogenation as the more stable the molecule less value for heat of hydrogenation.
Option B) This option is correct as this is 1,4 cyclohexadiene. It is a non-conjugated diene as the double bonds do not lie in conjugation thus it is least stable due to which maximum value for heat of hydrogenation.
Option C) this is an incorrect option.
Option D) this is an incorrect option as all the options will have different values for heat of hydrogenation depending upon the stability.
Note:
We have to remember that hydrogenation is nothing but chemical bond formation between hydrogen atoms and other molecules or compounds. The higher the double bond stability, minimum will be the value for heat of hydrogenation. Benzene is the most stable molecule with complete conjugation in it.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




