
Which functional group is present in a molecule of\[C{{H}_{3}}COOH\]?
(A) Ether
(B) Aldehyde
(C) Carboxyl
(D) Ester
(E) Amine
Answer
600.3k+ views
Hint: To answer this question, we should know about different functional groups in organic chemistry. The group that is present in this group is very common in biological molecules.
Complete step by step answer:
To answer this question, we should first know about the molecule that is given in question.
The structure of the molecule is as follows:
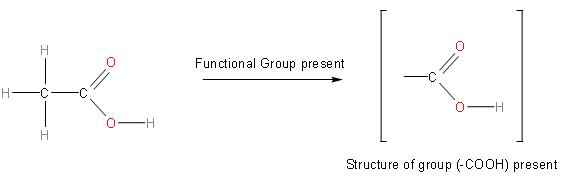 On the left hand side, this is the structure of acetic acid. And on the right hand side it is the group that is attached to the methyl group.So, one by one we will look at each and every option, and we will observe the structure of each group and then we will choose the correct option.So, the first option is either. Its structure is as follows:
On the left hand side, this is the structure of acetic acid. And on the right hand side it is the group that is attached to the methyl group.So, one by one we will look at each and every option, and we will observe the structure of each group and then we will choose the correct option.So, the first option is either. Its structure is as follows:

This structure doesn’t look like the structure that is present in the molecule of the given question. So, it is a wrong answer.
Now, we will look at the second option:
Our second option is aldehyde. Its structure is as follows:
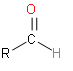
So, this structure also doesn’t look like the structure group that is attached to molecules.
Third option in the structure is the carboxyl group.
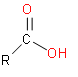
The above structure is of a carboxyl group. So, by looking at the structure above we can say that this structure looks similar to the structure present in the above question.
Now, we should also take a look at the structure of the ester group to confirm our answer.

This structure also doesn’t look like a structure of an acetic acid group.
In the last option Amine, the structure will contain atoms of nitrogen. So, amine is not our correct answer.
From the above discussion, we can say that the correct group that is present in the above question is the carboxyl group. And the structure present is of acetic acid. Our correct answer is option (C) carboxyl.
Note:
We should know that acetic acid is known by the common name of ethanoic acid. We should know that acetic acid comes in the application for making plastic soft drink bottles, photographic film; and polyvinyl acetate for wood glue, as well as many synthetic fibres and fabrics.
We should be careful in handling acetic acid. Because acetic acid liquid is highly corrosive to the skin and eyes and, because of this, must be handled with extreme care. Acetic acid can also be damaging to the internal organs if ingested or in the case of vapour inhalation.
Complete step by step answer:
To answer this question, we should first know about the molecule that is given in question.
The structure of the molecule is as follows:


This structure doesn’t look like the structure that is present in the molecule of the given question. So, it is a wrong answer.
Now, we will look at the second option:
Our second option is aldehyde. Its structure is as follows:

So, this structure also doesn’t look like the structure group that is attached to molecules.
Third option in the structure is the carboxyl group.

The above structure is of a carboxyl group. So, by looking at the structure above we can say that this structure looks similar to the structure present in the above question.
Now, we should also take a look at the structure of the ester group to confirm our answer.

This structure also doesn’t look like a structure of an acetic acid group.
In the last option Amine, the structure will contain atoms of nitrogen. So, amine is not our correct answer.
From the above discussion, we can say that the correct group that is present in the above question is the carboxyl group. And the structure present is of acetic acid. Our correct answer is option (C) carboxyl.
Note:
We should know that acetic acid is known by the common name of ethanoic acid. We should know that acetic acid comes in the application for making plastic soft drink bottles, photographic film; and polyvinyl acetate for wood glue, as well as many synthetic fibres and fabrics.
We should be careful in handling acetic acid. Because acetic acid liquid is highly corrosive to the skin and eyes and, because of this, must be handled with extreme care. Acetic acid can also be damaging to the internal organs if ingested or in the case of vapour inhalation.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




