
Which feature of the ammonia molecule leads to the formation of ammonium ion when ammonia dissolves in water?
Answer
595.8k+ views
Hint: We know that ammonia is a molecule consisting of one nitrogen atom and three hydrogen atoms. There is one lone pair of electrons present in the molecule of ammonia.
Complete step-by-step answer:
We first draw the molecular structure of ammonia molecules.
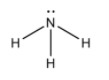
From the molecular structure, we see that, nitrogen atom forms three covalent bonds with hydrogen atoms. Also a lone pair of electrons is also present.
A base is a chemical compound compound that can easily donate a pair of electrons. As an ammonia molecule has one lone pair, it is a base.
When ammonia reacts with water ammonium ion produced. Nitrogen is a base which donates its lone pair to abstract protons from water molecules which produce ammonium ion and hydroxide ion. The mechanism of the reaction is shown below:
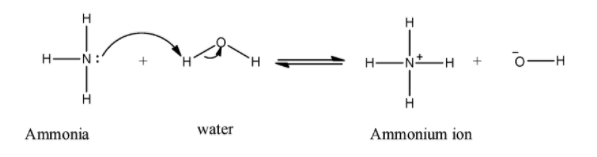
Hence, the basic feature of ammonia results in the ammonium ion when a molecule of ammonia reacts with water.
Additional Information:
Measurement of basicity: As most chemical species participate in reversible acid base reactions the position of equilibrium measures basicity. In other words we are measuring relative stability of the involved species. Stability is an example of thermodynamic property.
Note: Students might be confused in understanding acid and base. Donation of proton is by an acid and a base accepts proton. Here, in the ammonia molecule, due to the extra lone pair, it acts as a basic molecule and donates electrons to the abstract proton and thus forms ammonium ion.
Complete step-by-step answer:
We first draw the molecular structure of ammonia molecules.

From the molecular structure, we see that, nitrogen atom forms three covalent bonds with hydrogen atoms. Also a lone pair of electrons is also present.
A base is a chemical compound compound that can easily donate a pair of electrons. As an ammonia molecule has one lone pair, it is a base.
When ammonia reacts with water ammonium ion produced. Nitrogen is a base which donates its lone pair to abstract protons from water molecules which produce ammonium ion and hydroxide ion. The mechanism of the reaction is shown below:

Hence, the basic feature of ammonia results in the ammonium ion when a molecule of ammonia reacts with water.
Additional Information:
Measurement of basicity: As most chemical species participate in reversible acid base reactions the position of equilibrium measures basicity. In other words we are measuring relative stability of the involved species. Stability is an example of thermodynamic property.
Note: Students might be confused in understanding acid and base. Donation of proton is by an acid and a base accepts proton. Here, in the ammonia molecule, due to the extra lone pair, it acts as a basic molecule and donates electrons to the abstract proton and thus forms ammonium ion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




