
Which compound is used for bleaching clothes in the laundry?
(A) Bleaching powder
(B) Washing powder
(C) Baking powder
(D) Plaster of Paris
Answer
585.6k+ views
Hint: bleaching is a process used for the removal of colouring matter by converting into the colourless matter. The substrate is oxidized by the bleaching agent. The bleaching agent is capable of supplying the chlorine. The chlorine on the action of acid generates the nascent oxygen which oxides the clothes in the laundry.
Complete step by step answer:
Bleaching is a chemical treatment used for the removal of colouring matters from the substrate. In the laundry, the bleaching agent is a chemical treatment to whiten the clothes or decolorized clothes.
A bleaching agent is a chemical that whitens or decolorizes the fabric. This destroys the chromophore (Colour bearing groups) through the oxidation or the reduction of these groups.
One of the common bleaching agents is calcium hypochlorite$\text{ CaO(Cl}{{\text{)}}_{\text{2}}}\text{ }$. It is an inorganic substance and it’s a main component of the commercially used bleaching powder, chlorine powder, or chlorinated lime for the water treatment and as a bleaching agent. The $\text{ CaO(Cl}{{\text{)}}_{\text{2}}}\text{ }$ is a stable compound and it supplies the oxygen for the bleaching action.
The $\text{ CaO(Cl}{{\text{)}}_{\text{2}}}\text{ }$ is an oxidizing agent that produces oxygen for bleaching.
The bleaching powder when treated with the dilute acid produces the nascent oxygen $\text{ }\left[ \text{O} \right]\text{ }$. The nascent oxygen oxidizes the colouring matter and whitens the substrate.
The structure of the bleaching powder is as shown below:
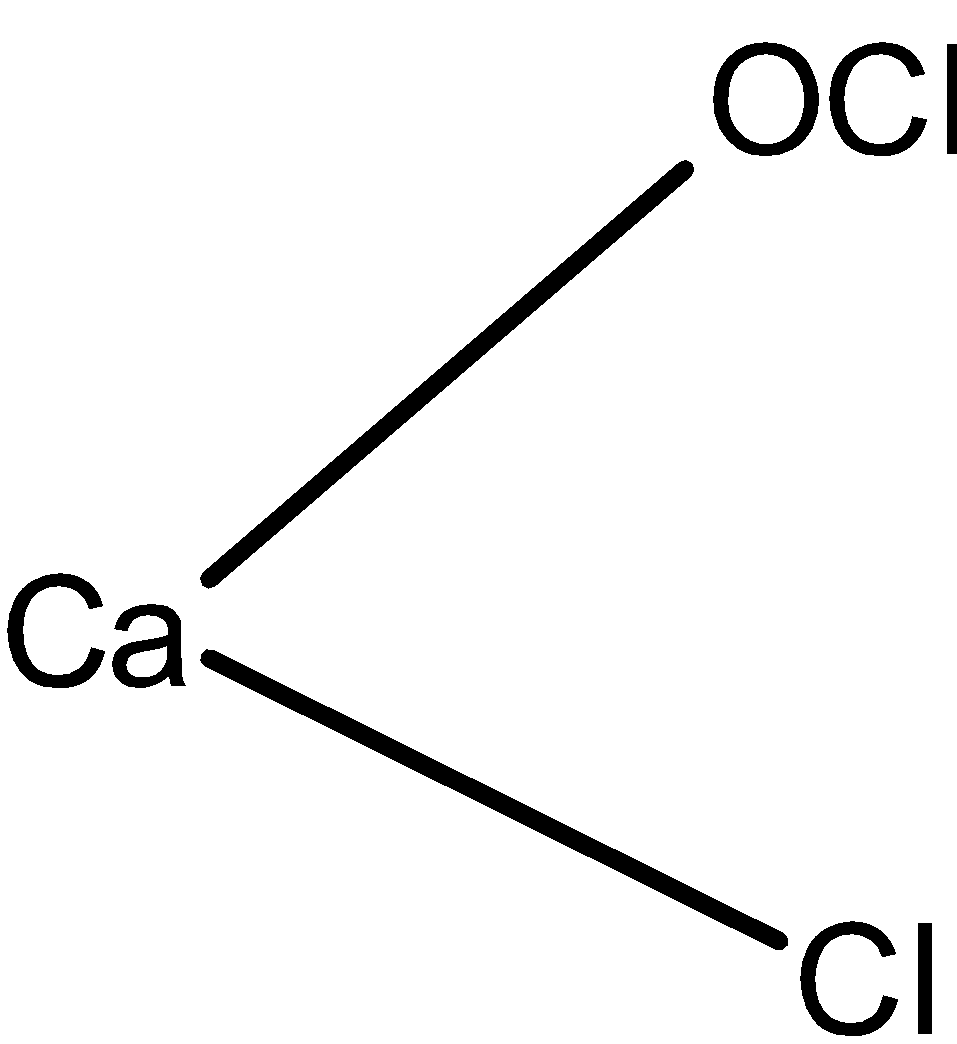
The $\text{ CaO(Cl}{{\text{)}}_{\text{2}}}\text{ }$calcium hypochlorite is also known as the bleaching powder.
Hence, (A) is the correct option.
Additional information:
The bleaching action of the calcium hypochlorite is as shown below:
The bleaching power $\text{ CaO(Cl}{{\text{)}}_{\text{2}}}\text{ }$is a mixed salt of hydrochloric acid and hypochlorous acid. When bleaching powder is treated with the dilute aid, it gives the nascent oxygen $\text{ }\left[ \text{O} \right]\text{ }$.the reaction is as shown below,
\[\text{ }\begin{array}{*{35}{l}}
\text{2CaOC}{{\text{l}}_{\text{2}}}\text{ + }{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ }\to \text{ CaC}{{\text{l}}_{\text{2}}}\text{ + CaS}{{\text{O}}_{\text{4}}}\text{ + 2HOCl} \\
2\text{HOCl }\to \text{ 2 HCl + 2 }\left[ \text{O} \right] \\
\end{array}\text{ }\]
The$\text{ CaO(Cl}{{\text{)}}_{\text{2}}}\text{ }$, when treated with the dilute acid, \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\] produces hypochlorous acid \[\text{HOCl}\]. The hypochlorous acid undergoes further dissociation to form hydrochloric acid and nascent oxygen. The nascent oxygen acts as an oxidizing agent.
$\text{ Color material + }\left[ \text{O} \right]\text{ }\to \text{ Colourless }$
This oxidizes the colouring matter and whitens the fabric.
Note: some of the common uses of bleaching powder are as follows:
1) The bleaching powder is used in the laundry for the bleaching of dirty clothes.it acts as a bleaching agent for cotton and linen in the textile industries.
2) The bleaching powder acts as an oxidizing agent in various industries.
3) The bleaching powder is act as a disinfectant to disinfect the water and make it safe to consume (potable water)
Complete step by step answer:
Bleaching is a chemical treatment used for the removal of colouring matters from the substrate. In the laundry, the bleaching agent is a chemical treatment to whiten the clothes or decolorized clothes.
A bleaching agent is a chemical that whitens or decolorizes the fabric. This destroys the chromophore (Colour bearing groups) through the oxidation or the reduction of these groups.
One of the common bleaching agents is calcium hypochlorite$\text{ CaO(Cl}{{\text{)}}_{\text{2}}}\text{ }$. It is an inorganic substance and it’s a main component of the commercially used bleaching powder, chlorine powder, or chlorinated lime for the water treatment and as a bleaching agent. The $\text{ CaO(Cl}{{\text{)}}_{\text{2}}}\text{ }$ is a stable compound and it supplies the oxygen for the bleaching action.
The $\text{ CaO(Cl}{{\text{)}}_{\text{2}}}\text{ }$ is an oxidizing agent that produces oxygen for bleaching.
The bleaching powder when treated with the dilute acid produces the nascent oxygen $\text{ }\left[ \text{O} \right]\text{ }$. The nascent oxygen oxidizes the colouring matter and whitens the substrate.
The structure of the bleaching powder is as shown below:

The $\text{ CaO(Cl}{{\text{)}}_{\text{2}}}\text{ }$calcium hypochlorite is also known as the bleaching powder.
Hence, (A) is the correct option.
Additional information:
The bleaching action of the calcium hypochlorite is as shown below:
The bleaching power $\text{ CaO(Cl}{{\text{)}}_{\text{2}}}\text{ }$is a mixed salt of hydrochloric acid and hypochlorous acid. When bleaching powder is treated with the dilute aid, it gives the nascent oxygen $\text{ }\left[ \text{O} \right]\text{ }$.the reaction is as shown below,
\[\text{ }\begin{array}{*{35}{l}}
\text{2CaOC}{{\text{l}}_{\text{2}}}\text{ + }{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ }\to \text{ CaC}{{\text{l}}_{\text{2}}}\text{ + CaS}{{\text{O}}_{\text{4}}}\text{ + 2HOCl} \\
2\text{HOCl }\to \text{ 2 HCl + 2 }\left[ \text{O} \right] \\
\end{array}\text{ }\]
The$\text{ CaO(Cl}{{\text{)}}_{\text{2}}}\text{ }$, when treated with the dilute acid, \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\] produces hypochlorous acid \[\text{HOCl}\]. The hypochlorous acid undergoes further dissociation to form hydrochloric acid and nascent oxygen. The nascent oxygen acts as an oxidizing agent.
$\text{ Color material + }\left[ \text{O} \right]\text{ }\to \text{ Colourless }$
This oxidizes the colouring matter and whitens the fabric.
Note: some of the common uses of bleaching powder are as follows:
1) The bleaching powder is used in the laundry for the bleaching of dirty clothes.it acts as a bleaching agent for cotton and linen in the textile industries.
2) The bleaching powder acts as an oxidizing agent in various industries.
3) The bleaching powder is act as a disinfectant to disinfect the water and make it safe to consume (potable water)
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




