
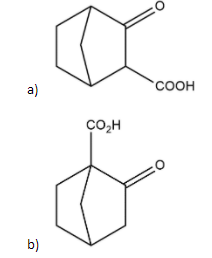
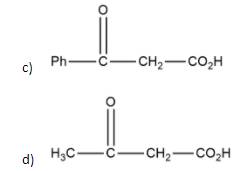
Which \[\beta \]- keto acid shown will not undergo decarboxylation?


Answer
591.6k+ views
Hint: Keto acids are also called oxo acids. These are organic compounds that contain a carboxylic acid group and a ketone group. There are 3 types of keto acids alpha, beta and gamma keto acids.
Complete step by step answer:
Compounds which contain carboxylic \[COOH\]and ketone group \[C=O\]present in it are known as keto acids or oxo acids. On the basis of attachment of ketone groups these are divided into 3 parts.
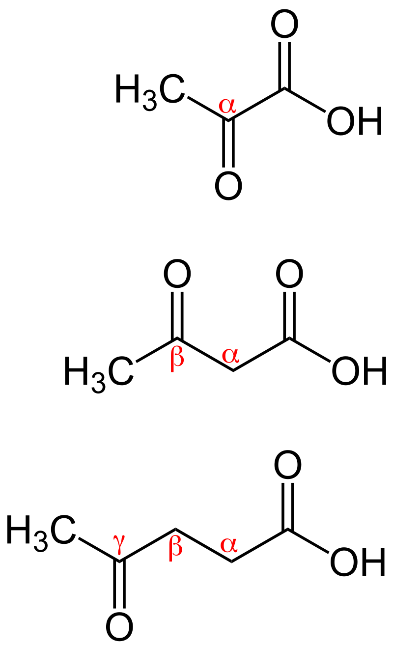
\[\alpha \]- keto acid in which ketone group is present at alpha carbon atom
\[\beta \]- keto acid in which ketone group is present at beta carbon atom
γ - keto acid in which ketone group is present at gamma carbon atom which can be shown as:
Decarboxylation takes place when carboxylic acids containing a carbonyl group two bonds away (on the \[\beta \]carbon) are heated and carbon dioxide is lost during this process.
In the case of compound b, \[\alpha \]\[\beta \]−keto acid having it's \[\alpha \]carbon at bridged head so it can't undergo tautomerization and it has a fixed hybridisation of \[s{{p}^{3}}\] and can't undergo decarboxylation while on the other compounds \[\beta \] position is free so decarboxylation can be occur easily.
Thus, option b is right.
Note: Decarboxylation is the process in which loss of carbon dioxide is there in this process of tautomerization. Tautomerism is the ability of certain organic compounds to react in isomeric structures that differ from each other in the position of a hydrogen atom and a double bond.
Complete step by step answer:
Compounds which contain carboxylic \[COOH\]and ketone group \[C=O\]present in it are known as keto acids or oxo acids. On the basis of attachment of ketone groups these are divided into 3 parts.
\[\alpha \]- keto acid in which ketone group is present at alpha carbon atom
\[\beta \]- keto acid in which ketone group is present at beta carbon atom
γ - keto acid in which ketone group is present at gamma carbon atom which can be shown as:

Decarboxylation takes place when carboxylic acids containing a carbonyl group two bonds away (on the \[\beta \]carbon) are heated and carbon dioxide is lost during this process.
In the case of compound b, \[\alpha \]\[\beta \]−keto acid having it's \[\alpha \]carbon at bridged head so it can't undergo tautomerization and it has a fixed hybridisation of \[s{{p}^{3}}\] and can't undergo decarboxylation while on the other compounds \[\beta \] position is free so decarboxylation can be occur easily.
Thus, option b is right.
Note: Decarboxylation is the process in which loss of carbon dioxide is there in this process of tautomerization. Tautomerism is the ability of certain organic compounds to react in isomeric structures that differ from each other in the position of a hydrogen atom and a double bond.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




