
Which are linear molecules? (This question has multiple correct options)
A.$C{O_2}$
B.${C_2}{H_2}$
C.$HCN$
D.${H_2}O$
Answer
585.3k+ views
Hint: We find hybridisation of each central atom by calculating lone pairs and bond pairs. And in case of Hydrocarbon, total sigma bonds. Now if hybridisation is ‘sp’ then that means angle between atoms is \[{180^0}\] and thus the structure is linear. Or there may be an arrangement of bond pair and lone pair in such a way that the angle between bond pairs becomes \[{180^0}\] which means it becomes a linear structure.
Complete step by step answer:
We try to find Hybridisation by finding lone pair and bond pair, thus determine bond angles and then try to draw shape and conclude about structure.
A. $C{O_2}$
Carbon has 4 electrons in the valence shell, shares 2 electrons with each oxygen atom, thus forming 2 sigma bonds and 2 pi bonds.
Hybrid orbitals = no. of sigma bonds = 2
So hybridisation is ‘sp’.
Thus it has linear structure.
Structure can be drawn as below:
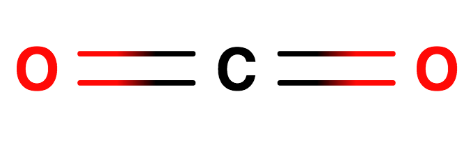
B.${C_2}{H_2}$
This is the formula of ethyne which is alkyne, which means triple bond between carbon-carbon atoms.
So, each carbon has 2 sigma bonds, one with another carbon and one with hydrogen.
Hybrid orbitals = number of sigma bonds = 2
Thus, hybridisation is ‘sp’ and bond angle is \[{180^0}\] and structure is linear.
Structure can be drawn as below:
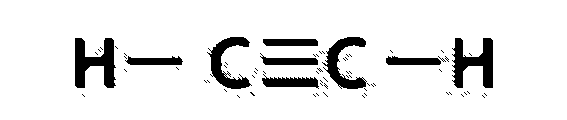
C.$HCN$
The carbon here is a central atom, and forms one sigma bond with hydrogen and triple bond with nitrogen, out of which 1 is sigma bond and 2 are pi bonds.
Thus this carbon has 2 sigma bonds, and hybrid orbitals = number of sigma bonds = 2
Thus hybridisation is ‘sp’ and bond angle is \[{180^0}\] and structure is linear.
Structure can be drawn as below:
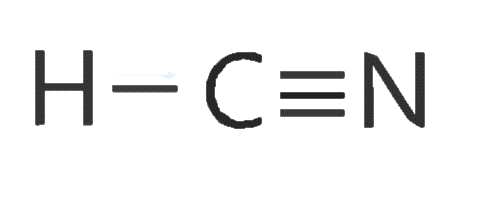
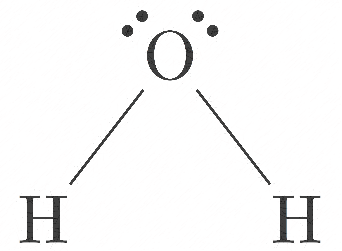
D.${H_2}O$
Here, oxygen has 6 valence electrons, so 2 electrons bonded with hydrogen (as it is a monovalent atom), and remaining 4 are with oxygen as 2 lone pairs.
Hybrid orbitals= 2 sigma bond + 2 lone pair = 4
So hybridisation is \[s{p^3}\] , but due to the presence of 2 lone pairs, the structure is bent.
The structure is shown below:
So, finally the correct answer which has linear structure is (A), (B) and (C).
Note:
There is a shortcut alternative to find hybridisation with one central atom.
Hybrid orbitals = \[\dfrac{1}{2}\] [valence electrons of central atom + monovalent atoms + magnitude of negative charge on central atom – magnitude of positive charge on central atom]
Complete step by step answer:
We try to find Hybridisation by finding lone pair and bond pair, thus determine bond angles and then try to draw shape and conclude about structure.
A. $C{O_2}$
Carbon has 4 electrons in the valence shell, shares 2 electrons with each oxygen atom, thus forming 2 sigma bonds and 2 pi bonds.
Hybrid orbitals = no. of sigma bonds = 2
So hybridisation is ‘sp’.
Thus it has linear structure.
Structure can be drawn as below:
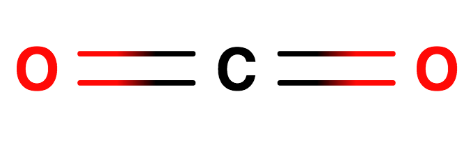
B.${C_2}{H_2}$
This is the formula of ethyne which is alkyne, which means triple bond between carbon-carbon atoms.
So, each carbon has 2 sigma bonds, one with another carbon and one with hydrogen.
Hybrid orbitals = number of sigma bonds = 2
Thus, hybridisation is ‘sp’ and bond angle is \[{180^0}\] and structure is linear.
Structure can be drawn as below:
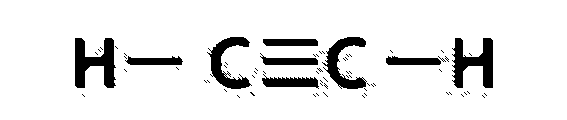
C.$HCN$
The carbon here is a central atom, and forms one sigma bond with hydrogen and triple bond with nitrogen, out of which 1 is sigma bond and 2 are pi bonds.
Thus this carbon has 2 sigma bonds, and hybrid orbitals = number of sigma bonds = 2
Thus hybridisation is ‘sp’ and bond angle is \[{180^0}\] and structure is linear.
Structure can be drawn as below:
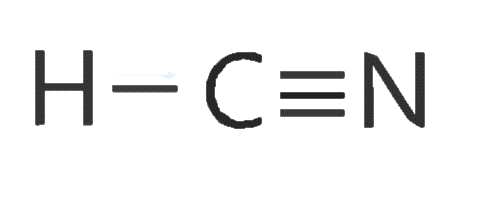
D.${H_2}O$
Here, oxygen has 6 valence electrons, so 2 electrons bonded with hydrogen (as it is a monovalent atom), and remaining 4 are with oxygen as 2 lone pairs.
Hybrid orbitals= 2 sigma bond + 2 lone pair = 4
So hybridisation is \[s{p^3}\] , but due to the presence of 2 lone pairs, the structure is bent.
The structure is shown below:

So, finally the correct answer which has linear structure is (A), (B) and (C).
Note:
There is a shortcut alternative to find hybridisation with one central atom.
Hybrid orbitals = \[\dfrac{1}{2}\] [valence electrons of central atom + monovalent atoms + magnitude of negative charge on central atom – magnitude of positive charge on central atom]
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




