
Where are neutrons located?
Answer
491.1k+ views
Hint: Neutrons are the subatomic particles of the atom which are neutral in charge and carry mass equal to that of protons. Neutrons are always located with protons. Both protons and neutrons are collectively known as nucleons. Thus neutrons are located with the protons inside the atom.
Complete answer:
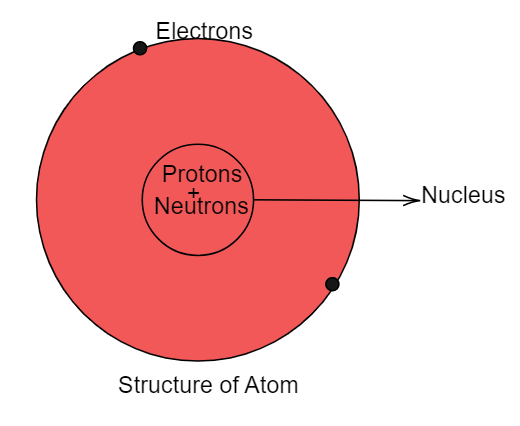
Atom is the smallest atomic particle of the element. There are subatomic particles which are present inside the atom. These subatomic particles are: electron, proton and neutron. Electrons can be found revolving around the nucleus of atoms in their fixed shell. Protons and neutrons are present collectively inside the nucleus of an atom. The neutron is a neutral charged particle which carries mass equal to the mass of a proton. Therefore neutrons contribute to the atomic mass of the element. It does not contribute to the charge of the atom. Protons and neutrons are collectively known as nucleons. Neutrons are not found in atoms of hydrogen. Since the atomic number of hydrogen is one. Therefore it contains one electron and one proton thus contains no neutrons. Except that all elements contain neutrons which are located inside the nucleus of atoms. Neutrons are located with protons inside the nucleus of an atom as shown below:
Note:
Neutrons were discovered by James Chadwick in \[1932\]. The discovery of neutrons leads to better understanding of atomic mass of the atom. In a neutral atom the number of protons is equal to the number of electrons. In cation and anion the number of electrons and protons may change but the number of neutrons do not change, since neutrons do not contribute to charge of atoms. It only contributes to the mass of the atom.
Complete answer:
Atom is the smallest atomic particle of the element. There are subatomic particles which are present inside the atom. These subatomic particles are: electron, proton and neutron. Electrons can be found revolving around the nucleus of atoms in their fixed shell. Protons and neutrons are present collectively inside the nucleus of an atom. The neutron is a neutral charged particle which carries mass equal to the mass of a proton. Therefore neutrons contribute to the atomic mass of the element. It does not contribute to the charge of the atom. Protons and neutrons are collectively known as nucleons. Neutrons are not found in atoms of hydrogen. Since the atomic number of hydrogen is one. Therefore it contains one electron and one proton thus contains no neutrons. Except that all elements contain neutrons which are located inside the nucleus of atoms. Neutrons are located with protons inside the nucleus of an atom as shown below:

Note:
Neutrons were discovered by James Chadwick in \[1932\]. The discovery of neutrons leads to better understanding of atomic mass of the atom. In a neutral atom the number of protons is equal to the number of electrons. In cation and anion the number of electrons and protons may change but the number of neutrons do not change, since neutrons do not contribute to charge of atoms. It only contributes to the mass of the atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




