
How many and what types of bonds are present in $NH_4^ + $?
A. 4 Covalent Bonds
B. 3 Covalent Bonds and Ionic Bond
C. 4 Ionic Bonds
D. 3 Covalent Bonds and 1 Co ordinate Bond
Answer
497.1k+ views
Hint: Chemical bonds are primarily of four types which are formed by atoms or molecules in order to yield a compound. These types of chemical bond include ionic bond, covalent bond, hydrogen bond and polar bond. Ionic bond involves transfer of electrons from one atom or molecule to another, whereas covalent bond indicates sharing of electrons between atoms.
Complete answer:
$N{H_4}^ + $contains three covalent and one coordinate bond.
A coordinate bond which is also known as a dative covalent bond and dipolar bond is a type of two- centered and two- electron covalent bond where both electrons come from the same or single atom. In coordinate bonds the atom that shares a pair of electrons from itself is called donor whereas, another atom which accepts the shared pair of electrons is known as a receptor or an acceptor.
In $N{H_4}^ + $the fourth hydrogen containing a vacant orbital is attached to the nitrogen atom containing a lone pair of electrons by a dative covalent bond or coordinate bond, thereby making nitrogen atom a donor and hydrogen atom an acceptor. Thus, satisfying the condition for the formation of a coordinate bond.
A covalent bond is a type of bond which is formed by sharing an equal number of electrons from both the participating atoms. The pair of electrons participating in this type of bonding is called a shared pair or bonding pair.
$N{H_4}^ + $contains three single covalent $N - H$ bonds where one electron is shared by both nitrogen atom and hydrogen atom.
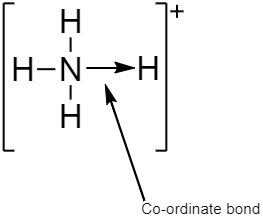
So the correct answer is option D.
Note:
$N{H_4}^ + $ is a positively charged polyatomic ion, which is the chemical formula of ammonium cation, which is formed by the protonation of ammonia. The hybridization of ammonium cation is $s{p^3}$ with tetrahedral geometry. The properties of ammonium ions are similar to the heavier alkali metals cations.
Complete answer:
$N{H_4}^ + $contains three covalent and one coordinate bond.
A coordinate bond which is also known as a dative covalent bond and dipolar bond is a type of two- centered and two- electron covalent bond where both electrons come from the same or single atom. In coordinate bonds the atom that shares a pair of electrons from itself is called donor whereas, another atom which accepts the shared pair of electrons is known as a receptor or an acceptor.
In $N{H_4}^ + $the fourth hydrogen containing a vacant orbital is attached to the nitrogen atom containing a lone pair of electrons by a dative covalent bond or coordinate bond, thereby making nitrogen atom a donor and hydrogen atom an acceptor. Thus, satisfying the condition for the formation of a coordinate bond.
A covalent bond is a type of bond which is formed by sharing an equal number of electrons from both the participating atoms. The pair of electrons participating in this type of bonding is called a shared pair or bonding pair.
$N{H_4}^ + $contains three single covalent $N - H$ bonds where one electron is shared by both nitrogen atom and hydrogen atom.

So the correct answer is option D.
Note:
$N{H_4}^ + $ is a positively charged polyatomic ion, which is the chemical formula of ammonium cation, which is formed by the protonation of ammonia. The hybridization of ammonium cation is $s{p^3}$ with tetrahedral geometry. The properties of ammonium ions are similar to the heavier alkali metals cations.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




