
What is urotropine? Give its uses.
Answer
604.5k+ views
HINT: It is a nitrogenous compound, also known as hexamine with a cage like structure similar to adamantane. It contains 4 molecules of nitrogen in each corner of its tetrahedral structure. It is used for various purposes in different industries like food additives, explosives and many more.
Complete step by step solution:
> Firstly, we will discuss what urotropine is and its structure-
- Urotropine is a white crystalline organic compound with the formula${{(C{{H}_{2}})}_{6}}{{N}_{4}}$. It is highly soluble in water and other polar organic solvents.
- It is also known as hexamethylenetetramine or methenamine.
- It is prepared industrially by condensation reaction of formaldehyde and ammonia, generally in a 4:6 ratio.
$4HCHO+6N{{H}_{3}}\to $
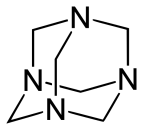 + $6{{H}_{2}}O$
+ $6{{H}_{2}}O$
4 moles of formaldehyde reacts with 6 moles of ammonia to give 1 mole of urotropine and 6 moles of water.
> As we can see in the above reaction, urotropine molecule has a symmetric tetrahedral cage like structure where the 4 corners are nitrogen atoms and the edges joining them are methylene ($C{{H}_{2}}$) bridges.
> USES OF UROTROPINE: Now we will discuss its uses. It is used in a variety of ways in many different industries, as discussed below-
1) Industrial Use- It is dominantly used as a hardening component in production of phenolic resins and phenolic resins moulding compounds.
2) Medicinal use- It is particularly used for long-term prophylactic treatment of urinary tract infection.
- Also used as a treatment for excessive sweating in the form of powder or cream.
3) Histological Stains- Hexamine silver stains are used for staining in histology like –
- Grocott’s methenamine silver stain, used to screen fungal organisms.
- Jones’ stain, stains for basement membranes that helps viewing the glomerular basement membrane.
4) Solid Fuel- Urotropine is a component of hexamine fuel tablets which has a high energy density and burns without smoke and leaves no ashes. It is often used for heating camping foods or military rations.
5) Standard tablets of urotropine are used by fire-protections laboratories as a fire source to test flammability of rugs and carpets.
6) It is also used as a food additive as a preservative.
7) It is a common reagent for organic synthesis like the Sommelet reaction (converting benzyl halides to aldehydes), Duff Reaction(formylation of arenes) and in the Delepine Reaction(synthesis of amines from alkyl halides).
8) It is also the base component for the production of RDX (a more energetic explosive than TNT), C-4 (a plastic explosive family based on RDX) and many more of such powerful explosives.
Additional Information:
Urotropine was also used as a method of treatment for soldiers exposed to phosgene in World War I. Studies have shown that large doses of urotropine provide some protection if taken before phosgene exposure but none if taken afterwards.
In an alkaline environment, It is completely inactive. Therefore, its effectiveness as a urinary antiseptic is dependent on the acidity of the urine rather than the amount of drug administered.
NOTE: Its structure is similar to adamantane,${{C}_{10}}{{H}_{16}}$, but it has 4 nitrogen atoms present in 4 corners which make it different from adamantine.
Although it has a cage-like structure, there is no void present inside for binding with other atoms or molecules unlike other cryptands and crown ethers.
Complete step by step solution:
> Firstly, we will discuss what urotropine is and its structure-
- Urotropine is a white crystalline organic compound with the formula${{(C{{H}_{2}})}_{6}}{{N}_{4}}$. It is highly soluble in water and other polar organic solvents.
- It is also known as hexamethylenetetramine or methenamine.
- It is prepared industrially by condensation reaction of formaldehyde and ammonia, generally in a 4:6 ratio.
$4HCHO+6N{{H}_{3}}\to $

4 moles of formaldehyde reacts with 6 moles of ammonia to give 1 mole of urotropine and 6 moles of water.
> As we can see in the above reaction, urotropine molecule has a symmetric tetrahedral cage like structure where the 4 corners are nitrogen atoms and the edges joining them are methylene ($C{{H}_{2}}$) bridges.
> USES OF UROTROPINE: Now we will discuss its uses. It is used in a variety of ways in many different industries, as discussed below-
1) Industrial Use- It is dominantly used as a hardening component in production of phenolic resins and phenolic resins moulding compounds.
2) Medicinal use- It is particularly used for long-term prophylactic treatment of urinary tract infection.
- Also used as a treatment for excessive sweating in the form of powder or cream.
3) Histological Stains- Hexamine silver stains are used for staining in histology like –
- Grocott’s methenamine silver stain, used to screen fungal organisms.
- Jones’ stain, stains for basement membranes that helps viewing the glomerular basement membrane.
4) Solid Fuel- Urotropine is a component of hexamine fuel tablets which has a high energy density and burns without smoke and leaves no ashes. It is often used for heating camping foods or military rations.
5) Standard tablets of urotropine are used by fire-protections laboratories as a fire source to test flammability of rugs and carpets.
6) It is also used as a food additive as a preservative.
7) It is a common reagent for organic synthesis like the Sommelet reaction (converting benzyl halides to aldehydes), Duff Reaction(formylation of arenes) and in the Delepine Reaction(synthesis of amines from alkyl halides).
8) It is also the base component for the production of RDX (a more energetic explosive than TNT), C-4 (a plastic explosive family based on RDX) and many more of such powerful explosives.
Additional Information:
Urotropine was also used as a method of treatment for soldiers exposed to phosgene in World War I. Studies have shown that large doses of urotropine provide some protection if taken before phosgene exposure but none if taken afterwards.
In an alkaline environment, It is completely inactive. Therefore, its effectiveness as a urinary antiseptic is dependent on the acidity of the urine rather than the amount of drug administered.
NOTE: Its structure is similar to adamantane,${{C}_{10}}{{H}_{16}}$, but it has 4 nitrogen atoms present in 4 corners which make it different from adamantine.
Although it has a cage-like structure, there is no void present inside for binding with other atoms or molecules unlike other cryptands and crown ethers.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




