
What is the triplet point?

Answer
582.3k+ views
Hint: The temperature and pressure at which all three phases coexist is known as triplet point. Triplet point is unique for every substance.
Step by step answer: The given diagrams is represented as follows:
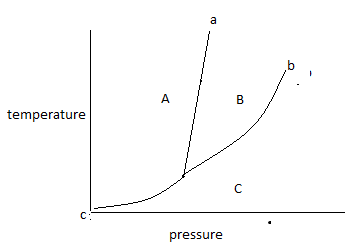
The above diagram is known as a phase diagram.
The phase diagram shows the temperature and pressure conditions for the conversion of different phases into each other and the triple point at which all the phases coexist. The phase diagram is plotted between temperature and pressure.
The given phase diagram is for one component system.
The phase diagram contains areas, curves, and triplet points.
In the above phase diagram, area A is for the solid phase, area B is for the liquid phase and area C is for the gas phase. Area shows the existence of one phase only in a temperature and pressure value. On changing, temperature and pressure in the area phase does not change.
Curve ‘a’ represents the fusion curve, curve ‘b’ represents the vaporization curve and curve ‘c’ represents the sublimation curve. The curve shows the existence of two phases at equilibrium at the temperature and pressure values. Any temperature and pressure values at the curve, two phases coexist.
The fusion curve shows the coexistence of solid and liquid.
The vaporization curve shows the coexistence of liquid and gas.
The sublimation curve shows the coexistence of solid and gas.
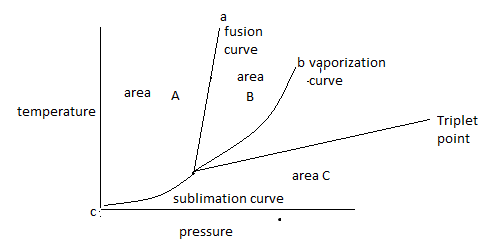
Triplet point is the point at which three curves a, b and c meet with each other. Therefore, the meeting point of all curves in the diagram shows the triplet point.
Note: Triplet point can be identified in the diagram as the point at which three curves join each other. At a triplet point all three phases’ remains in equilibrium. Some substances do not coexist in all three phases at any temperature and pressure value, that substance does not have a triple point.
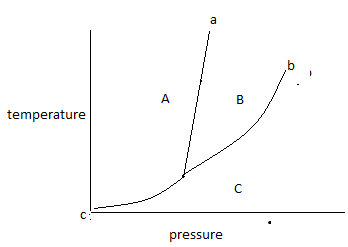
Step by step answer: The given diagrams is represented as follows:

The above diagram is known as a phase diagram.
The phase diagram shows the temperature and pressure conditions for the conversion of different phases into each other and the triple point at which all the phases coexist. The phase diagram is plotted between temperature and pressure.
The given phase diagram is for one component system.
The phase diagram contains areas, curves, and triplet points.
In the above phase diagram, area A is for the solid phase, area B is for the liquid phase and area C is for the gas phase. Area shows the existence of one phase only in a temperature and pressure value. On changing, temperature and pressure in the area phase does not change.
Curve ‘a’ represents the fusion curve, curve ‘b’ represents the vaporization curve and curve ‘c’ represents the sublimation curve. The curve shows the existence of two phases at equilibrium at the temperature and pressure values. Any temperature and pressure values at the curve, two phases coexist.
The fusion curve shows the coexistence of solid and liquid.
The vaporization curve shows the coexistence of liquid and gas.
The sublimation curve shows the coexistence of solid and gas.

Triplet point is the point at which three curves a, b and c meet with each other. Therefore, the meeting point of all curves in the diagram shows the triplet point.
Note: Triplet point can be identified in the diagram as the point at which three curves join each other. At a triplet point all three phases’ remains in equilibrium. Some substances do not coexist in all three phases at any temperature and pressure value, that substance does not have a triple point.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




