
What is the thermite process?
Answer
588.9k+ views
Hint: Mixture of aluminium (Al) powder and ferric oxide ($F{{e}_{2}}{{O}_{3}}$) is known as thermite. On igniting this mixture, aluminium is oxidized while ferric oxide is reduced to obtain iron metal. The reaction between Al powder and $F{{e}_{2}}{{O}_{3}}$ is highly exothermic.
Complete step by step solution:
The thermite process is explained below:
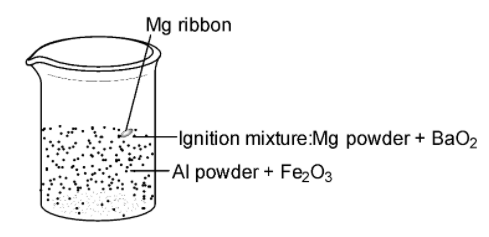
-A mixture of aluminium powder and ferric oxide, which is called thermite, is ignited. A burning magnesium ribbon is inserted into the thermite containing magnesium powder and barium peroxide ($Ba{{O}_{2}}$). The amount of heat produced due to this ignition mixture is sufficient to initiate the reaction.
-As aluminium is more reactive than iron, and also the free energy of formation of aluminium oxide, i.e. ${{\Delta }_{f}}{{G}^{o}}$ of $A{{l}_{2}}{{O}_{3}}$ is more negative than that of ferric oxide. Al is oxidized to $A{{l}_{2}}{{O}_{3}}$ and it reduces $F{{e}_{2}}{{O}_{3}}$ to Fe metal.
\[2Al(s)+F{{e}_{2}}{{O}_{3}}(s)\xrightarrow{\Delta }A{{l}_{2}}{{O}_{3}}(s)+Fe(l)\]
-Aluminum can also be used to reduce other metal oxides like chromium oxide ($C{{r}_{2}}{{O}_{3}}$), manganese oxide ($M{{n}_{2}}{{O}_{3}}$), etc.
-Therefore, we can define the thermite process as the process of reduction of iron oxide or any metal oxide using aluminium powder as the reducing agent. It is also commonly known as Goldschmidt thermite process or aluminothermy.
-The reaction produces a large amount of heat which is enough to attain the temperature of about 3500$^{o}C$. The iron thus obtained is in the molten state.
-This process is used in the welding of railway tracks, heavy machinery, etc. This is because the metallic iron produced in this process is melted down due to a large amount of heat released during the process. Hence, this melted iron can be used to join or weld broken iron articles, railway tracks, machines, etc.
Note: Aluminum is more reactive than iron, it means that aluminium undergoes oxidation more readily than iron. Therefore, it acts as a reducing agent and reduces iron (III) oxide to iron. It is to be noted that the reaction is only needed to be initiated as the oxidation of aluminium produces enough heat to further drive the process.
Complete step by step solution:
The thermite process is explained below:
-A mixture of aluminium powder and ferric oxide, which is called thermite, is ignited. A burning magnesium ribbon is inserted into the thermite containing magnesium powder and barium peroxide ($Ba{{O}_{2}}$). The amount of heat produced due to this ignition mixture is sufficient to initiate the reaction.
-As aluminium is more reactive than iron, and also the free energy of formation of aluminium oxide, i.e. ${{\Delta }_{f}}{{G}^{o}}$ of $A{{l}_{2}}{{O}_{3}}$ is more negative than that of ferric oxide. Al is oxidized to $A{{l}_{2}}{{O}_{3}}$ and it reduces $F{{e}_{2}}{{O}_{3}}$ to Fe metal.
\[2Al(s)+F{{e}_{2}}{{O}_{3}}(s)\xrightarrow{\Delta }A{{l}_{2}}{{O}_{3}}(s)+Fe(l)\]
-Aluminum can also be used to reduce other metal oxides like chromium oxide ($C{{r}_{2}}{{O}_{3}}$), manganese oxide ($M{{n}_{2}}{{O}_{3}}$), etc.

-Therefore, we can define the thermite process as the process of reduction of iron oxide or any metal oxide using aluminium powder as the reducing agent. It is also commonly known as Goldschmidt thermite process or aluminothermy.
-The reaction produces a large amount of heat which is enough to attain the temperature of about 3500$^{o}C$. The iron thus obtained is in the molten state.
-This process is used in the welding of railway tracks, heavy machinery, etc. This is because the metallic iron produced in this process is melted down due to a large amount of heat released during the process. Hence, this melted iron can be used to join or weld broken iron articles, railway tracks, machines, etc.
Note: Aluminum is more reactive than iron, it means that aluminium undergoes oxidation more readily than iron. Therefore, it acts as a reducing agent and reduces iron (III) oxide to iron. It is to be noted that the reaction is only needed to be initiated as the oxidation of aluminium produces enough heat to further drive the process.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




