
What is the shape of \[C{H_4}O\]?
Answer
502.8k+ views
Hint:Molecules are the combination of atoms or chemical elements. The atoms are attached through bonding. Methanol is a molecule with two geometric centres; one is oxygen and the other is carbon. The geometry of the molecule can be determined from the valence electrons and the hybridisation.
Complete answer:
Given molecule is methanol. Due to the presence of hydroxyl group, it comes under alcohols. The IUPAC nomenclature of alcohols has the suffix of -al. The molecular formula of methanol is \[C{H_4}O\]. It has one carbon atom, one oxygen atom and four hydrogen atoms. Thus, methanol has two geometric centres, one is oxygen and the other is carbon.
Carbon is an element with atomic number \[6\] and the valence electrons are \[4\].
Oxygen is an element with atomic number \[8\] and the valence electrons are \[6\].
Hydrogen is an element with atomic number \[1\] and the valence electrons from four hydrogen atoms are\[4\].
The total number of valence electrons from all the atoms in methanol are \[14\]. These \[14\] electrons are distributed between the atoms to form a covalent bond. The total electrons involved in bonding are \[10\]. The remaining four electrons exist as lone pairs on oxygen atoms.
The structure of methanol will be as follows:
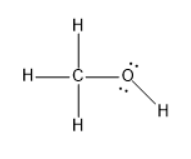
The carbon is tetrahedral geometry and the molecular geometry is also tetrahedral.
The oxygen has tetrahedral electron geometry and the molecular geometry is bent.
Note:
All the atoms in methanol are single bonded and have \[s{p^3}\] hybridization. Thus, the electron geometry will be a tetrahedron. But, the two lone pairs on an oxygen atom makes the oxygen atom to be bent in molecular geometry.
Complete answer:
Given molecule is methanol. Due to the presence of hydroxyl group, it comes under alcohols. The IUPAC nomenclature of alcohols has the suffix of -al. The molecular formula of methanol is \[C{H_4}O\]. It has one carbon atom, one oxygen atom and four hydrogen atoms. Thus, methanol has two geometric centres, one is oxygen and the other is carbon.
Carbon is an element with atomic number \[6\] and the valence electrons are \[4\].
Oxygen is an element with atomic number \[8\] and the valence electrons are \[6\].
Hydrogen is an element with atomic number \[1\] and the valence electrons from four hydrogen atoms are\[4\].
The total number of valence electrons from all the atoms in methanol are \[14\]. These \[14\] electrons are distributed between the atoms to form a covalent bond. The total electrons involved in bonding are \[10\]. The remaining four electrons exist as lone pairs on oxygen atoms.
The structure of methanol will be as follows:

The carbon is tetrahedral geometry and the molecular geometry is also tetrahedral.
The oxygen has tetrahedral electron geometry and the molecular geometry is bent.
Note:
All the atoms in methanol are single bonded and have \[s{p^3}\] hybridization. Thus, the electron geometry will be a tetrahedron. But, the two lone pairs on an oxygen atom makes the oxygen atom to be bent in molecular geometry.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




