
What is the reverse Carnot Cycle?
Answer
503.4k+ views
Hint: Let us first get some idea about the Carnot Cycle. The Carnot cycle is a theoretical ideal thermodynamic cycle proposed by French scientist Nicolas Léonard Sadi Carnot in \[1824\] and further developed by others during the following decades. It establishes an upper limit on the efficiency of any classical thermodynamic engine in converting heat into work, or, conversely, the efficiency of a refrigeration system in producing a temperature difference by applying work to the system. It is a theoretical construct rather than a real thermodynamic cycle.
Complete step by step solution:
Let us know a little more about the carnot cycle. Every thermodynamic system exists in a specific state. A thermodynamic cycle occurs when a system is put through a succession of various states before being returned to its initial condition. The system may perform work on its surroundings while cycling through this cycle, for example, by moving a piston and serving as a heat engine. A Carnot heat engine is a system that goes through the Carnot cycle, albeit such a "perfect" engine is simply a theoretical construct that cannot be produced in practise.
The directions of heat and work interactions are reversed when the Carnot cycle is reversed. A Carnot refrigerator or heat pump is a refrigerator or heat pump that operates on the reversed Carnot cycle.
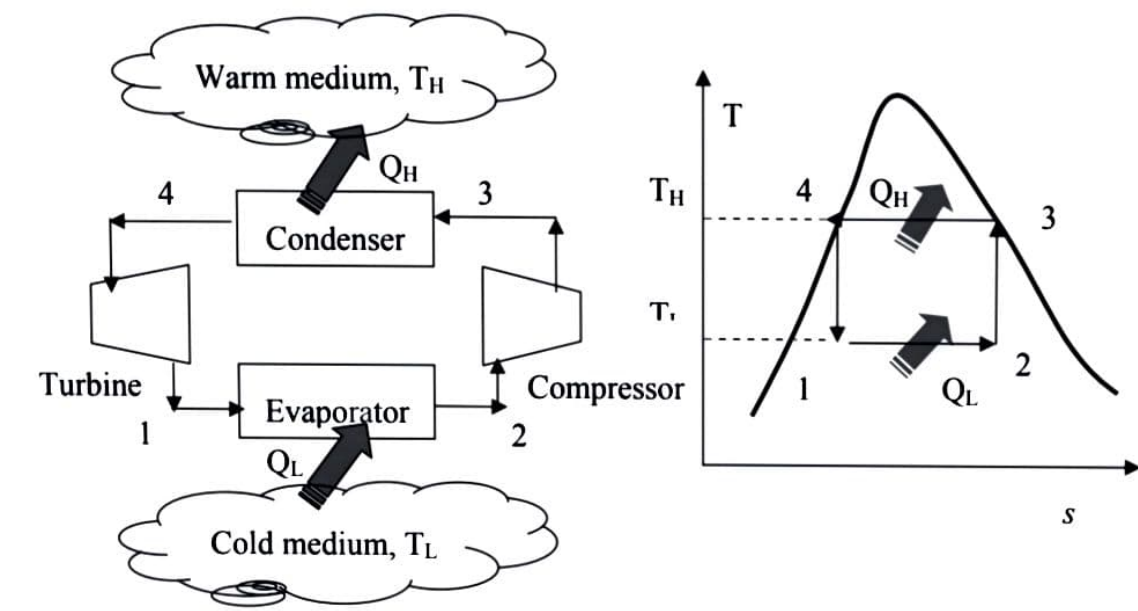
The reversed Carnot cycle, which operates between two temperature levels, is the most efficient refrigeration cycle. It establishes the theoretical maximum COP.
Note:
For a particular temperature limit, it has the highest efficiency. All four stages can be reversed because it is a reversible cycle. As a result, the direction of heat and work interactions will be reversed, resulting in a refrigeration cycle.
Complete step by step solution:
Let us know a little more about the carnot cycle. Every thermodynamic system exists in a specific state. A thermodynamic cycle occurs when a system is put through a succession of various states before being returned to its initial condition. The system may perform work on its surroundings while cycling through this cycle, for example, by moving a piston and serving as a heat engine. A Carnot heat engine is a system that goes through the Carnot cycle, albeit such a "perfect" engine is simply a theoretical construct that cannot be produced in practise.
The directions of heat and work interactions are reversed when the Carnot cycle is reversed. A Carnot refrigerator or heat pump is a refrigerator or heat pump that operates on the reversed Carnot cycle.

The reversed Carnot cycle, which operates between two temperature levels, is the most efficient refrigeration cycle. It establishes the theoretical maximum COP.
Note:
For a particular temperature limit, it has the highest efficiency. All four stages can be reversed because it is a reversible cycle. As a result, the direction of heat and work interactions will be reversed, resulting in a refrigeration cycle.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




