
What is the formal charge of ${ NO }_{ 2 }^{ - }$?
Answer
598.2k+ views
Hint: The formal charge is the difference in the number of valence electrons in the atom and the number of valence electrons in the Lewis structure.
Complete answer step-by-step:
The formal charge of an atom in a molecule can be calculated by the following equation;
${ FC=V-N-B\div 2 }$
where V= number of valence electrons of the atom in the free atom
N = number of nonbonding electrons on this atom in the molecule
B = Total number of electrons shared in covalent bonds with other atoms in the molecule.
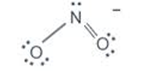
Nitrogen in the nitro group ${ NO }_{ 2 }^{ - }$ : FC= ${ 5-2-6\div 2=0 }$
Double bonded oxygen in ${ NO }_{ 2 }^{ - }$ : FC=${ 6-4-4\div 2=0 }$
Single bonded oxygen in ${ NO }_{ 2 }^{ - }$ : FC= ${ 6-6-2\div 2=-1 }$
Hence, ${ 0+0+(-1)=-1 }$ as expected for ${ NO }_{ 2 }^{ - }$
Additional Information:
Formal charge means ignore the lone pair of electrons and electrons shared by that atom from valence electrons then whatever is left with you is the significance of formal charge.
- It helps us to keep track of the electrons in a molecule.
- It tells you whether an atom has more electrons or protons associated with it.
- It allows us to assign electrons to a particular atom in a molecule.
Note: The possibility to make a mistake is that you may confuse between formal charge and the actual charge. But keep in mind that formal charges do not represent the actual charge on atoms in a molecule. The formal charges are assigned to the atoms in the Lewis structure to keep track of the electrons involved in the bonding.
Complete answer step-by-step:
The formal charge of an atom in a molecule can be calculated by the following equation;
${ FC=V-N-B\div 2 }$
where V= number of valence electrons of the atom in the free atom
N = number of nonbonding electrons on this atom in the molecule
B = Total number of electrons shared in covalent bonds with other atoms in the molecule.

Nitrogen in the nitro group ${ NO }_{ 2 }^{ - }$ : FC= ${ 5-2-6\div 2=0 }$
Double bonded oxygen in ${ NO }_{ 2 }^{ - }$ : FC=${ 6-4-4\div 2=0 }$
Single bonded oxygen in ${ NO }_{ 2 }^{ - }$ : FC= ${ 6-6-2\div 2=-1 }$
Hence, ${ 0+0+(-1)=-1 }$ as expected for ${ NO }_{ 2 }^{ - }$
Additional Information:
Formal charge means ignore the lone pair of electrons and electrons shared by that atom from valence electrons then whatever is left with you is the significance of formal charge.
- It helps us to keep track of the electrons in a molecule.
- It tells you whether an atom has more electrons or protons associated with it.
- It allows us to assign electrons to a particular atom in a molecule.
Note: The possibility to make a mistake is that you may confuse between formal charge and the actual charge. But keep in mind that formal charges do not represent the actual charge on atoms in a molecule. The formal charges are assigned to the atoms in the Lewis structure to keep track of the electrons involved in the bonding.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




