
What is the excited state of carbon?
Answer
513k+ views
Hint: An excited-state of an atom is a state of the atom in which the total energy of the electrons can be lowered by transferring one or more electrons to different orbitals which are higher in energy.
When we write the electronic configuration for carbon the first two electrons will go in the \[1s\] orbital. Since the maximum capacity of the \[1s\] orbital is 2, the next 2 electrons will go in the \[2s\] orbital. The remaining two electrons will go in the \[2p\] orbital. Therefore the electron configuration of the carbon atom will be \[1{s^2}2{s^2}2{p^2}\].
Complete answer:
The arrangement of electrons in the atomic orbitals of an atom is known as the electron configuration of that atom. Electron configurations are determined using the periodic table. Carbon is the sixth element in the periodic table with a total of 6 electrons. Hence the atomic number of carbon is 6.
From this and already mentioned hint we can write that
The ground state electron configuration of carbon is \[1{s^2}2{s^2}2{p^2}\]
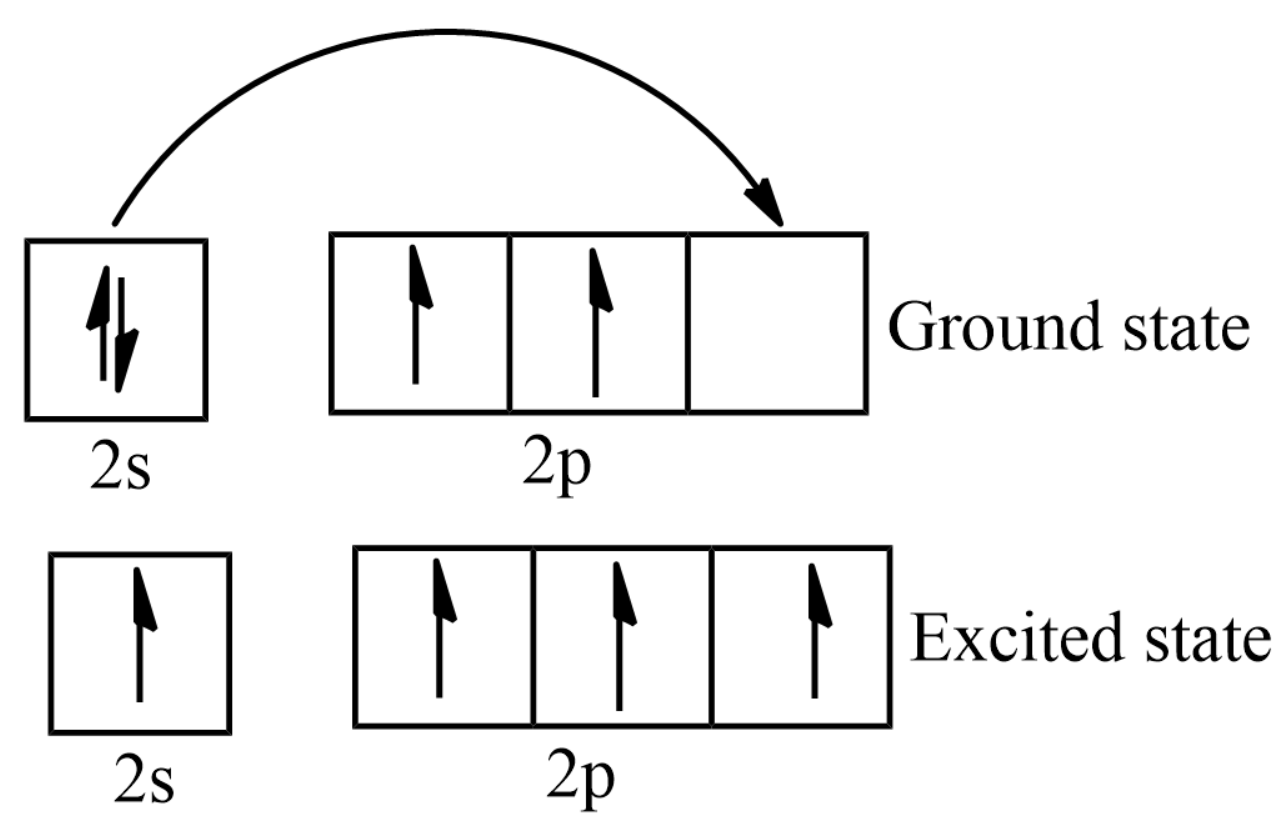
The total energy of the electrons in the carbon atom will be elevated by exciting an electron from the \[2s\] orbital to the \[2p\] orbital. Therefore, this carbon atom is in an excited-state.
Note:
The excited state is the state of carbon when it undergoes chemical bonding to form four covalent bonds, like in methane. However we have found out experimentally that all four bonds of methane have the same energy, which can only be explained by the concept that orbitals hybridize to form four \[s{p^3}\] orbitals, each with one unpaired electron and the same energy.
When we write the electronic configuration for carbon the first two electrons will go in the \[1s\] orbital. Since the maximum capacity of the \[1s\] orbital is 2, the next 2 electrons will go in the \[2s\] orbital. The remaining two electrons will go in the \[2p\] orbital. Therefore the electron configuration of the carbon atom will be \[1{s^2}2{s^2}2{p^2}\].
Complete answer:
The arrangement of electrons in the atomic orbitals of an atom is known as the electron configuration of that atom. Electron configurations are determined using the periodic table. Carbon is the sixth element in the periodic table with a total of 6 electrons. Hence the atomic number of carbon is 6.
From this and already mentioned hint we can write that
The ground state electron configuration of carbon is \[1{s^2}2{s^2}2{p^2}\]

The total energy of the electrons in the carbon atom will be elevated by exciting an electron from the \[2s\] orbital to the \[2p\] orbital. Therefore, this carbon atom is in an excited-state.
Note:
The excited state is the state of carbon when it undergoes chemical bonding to form four covalent bonds, like in methane. However we have found out experimentally that all four bonds of methane have the same energy, which can only be explained by the concept that orbitals hybridize to form four \[s{p^3}\] orbitals, each with one unpaired electron and the same energy.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




