
What is Fenton’s reagent?
Answer
504.6k+ views
Hint: We need to know that the reagent is a compound or it is a compound which is put on to the system to initiate the chemical reaction. If the reaction occurs, the reagent is added to the test. Both reagents and reactants are used interchangeably. Hence, the term reactant can be replaced by a reagent. But the reactant is more distinctly a substance or a compound will consume during a chemical reaction. The reactant is converted into another product by using a particular reagent during a chemical reaction.
Complete answer:
The Fenton’s reagent is a solution containing the hydrogen peroxide with ferrous ions. The ferrous ion in this reagent acts as a catalyst which is used to oxidize the waste waters or it is also used to oxidize the contaminants. By using the Fenton’s reagent, we can destroy the organic compounds like tetrachloroethylene and trichloroethylene. The molecular formula of Fenton’s reagent is
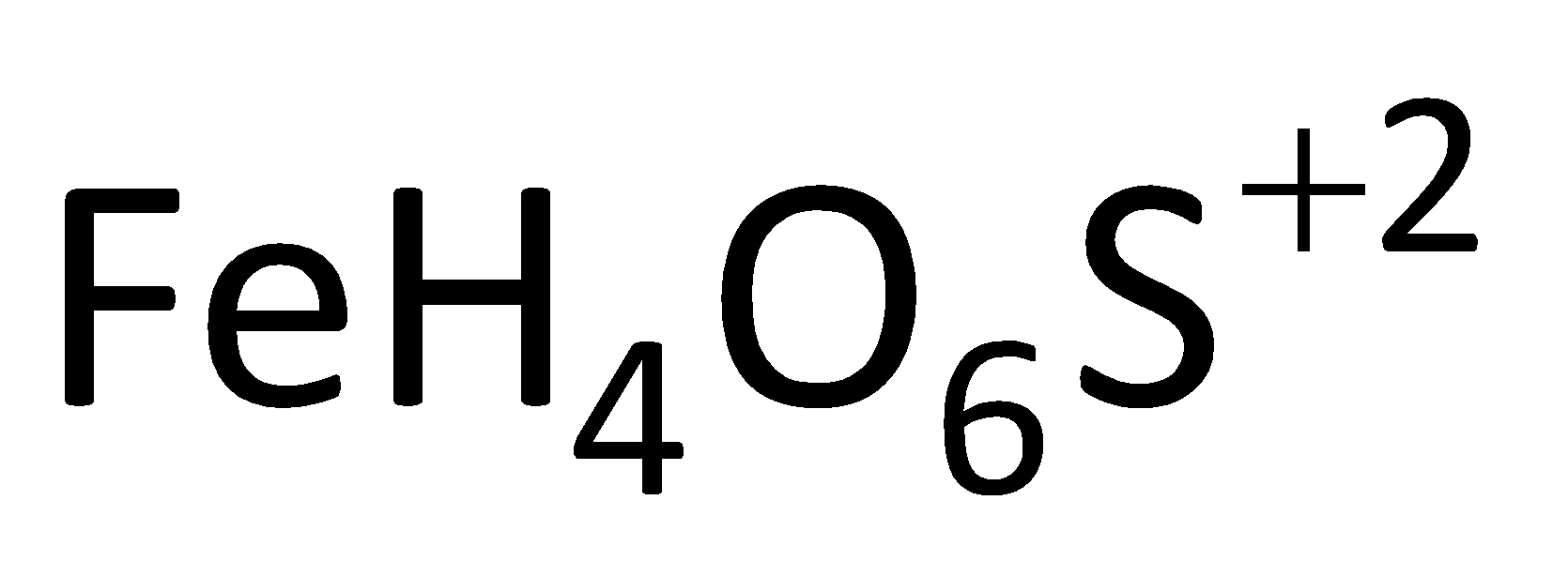 . And this reagent can be prepared by dissolving ferrous sulphate in sterilized deionized water in the presence of concentrated sulphuric acid at a definite amount. It is also used for the degradation of many compounds like phenol, formic acid, nitrobenzene etc. The ferrous ions present in the catalyst help to promote the formation of free radicals.
. And this reagent can be prepared by dissolving ferrous sulphate in sterilized deionized water in the presence of concentrated sulphuric acid at a definite amount. It is also used for the degradation of many compounds like phenol, formic acid, nitrobenzene etc. The ferrous ions present in the catalyst help to promote the formation of free radicals.
By using Fenton’s reagent we can convert benzene into phenol, barbituric acid convert into alloxan and it is also used for the hydroxylation of arenes.
Note:
We need to know that the Fenton’s reagent is the solution consisting of ferrous ions and hydrogen peroxide. In advanced oxidation method, widely using the Fenton’s reagent. In the time of Fenton – oxidation process, it consists of the activation of hydrogen peroxide by using the ferrous ion and there is a formation of hydroxyl radicals in the complex reaction under acidic conditions.
Complete answer:
The Fenton’s reagent is a solution containing the hydrogen peroxide with ferrous ions. The ferrous ion in this reagent acts as a catalyst which is used to oxidize the waste waters or it is also used to oxidize the contaminants. By using the Fenton’s reagent, we can destroy the organic compounds like tetrachloroethylene and trichloroethylene. The molecular formula of Fenton’s reagent is
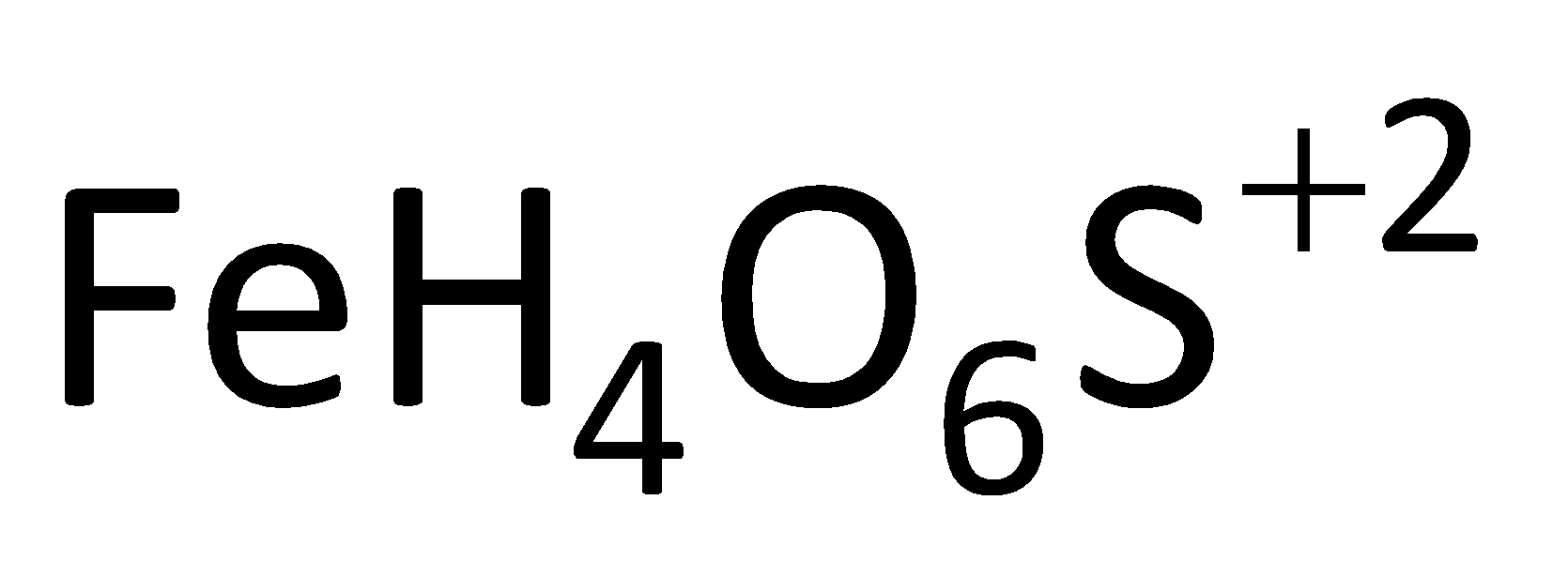
By using Fenton’s reagent we can convert benzene into phenol, barbituric acid convert into alloxan and it is also used for the hydroxylation of arenes.
Note:
We need to know that the Fenton’s reagent is the solution consisting of ferrous ions and hydrogen peroxide. In advanced oxidation method, widely using the Fenton’s reagent. In the time of Fenton – oxidation process, it consists of the activation of hydrogen peroxide by using the ferrous ion and there is a formation of hydroxyl radicals in the complex reaction under acidic conditions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




