
What is diazotization?
Answer
596.4k+ views
Hint:This reaction is based on the aromatic amines, when there is a reaction with the nitrous acid. From the name itself, we can say that the product obtained must be related to the diazonium salt.
Complete step by step solution:
> Let us define the diazotization. It is a chemical process involved in organic chemistry.
- It is the conversion of primary aromatic amine into the diazonium salt of amine by the use of nitrous acid; also known as diazotization.
- This reaction was discovered by Peter Griess, and he proposed many reactions having diazonium salts.
Now, we will see the reaction representing the diazotization is
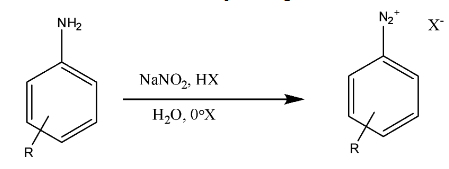
> We can see that this reaction represents the reaction of primary aromatic amines with sodium nitrite, and a strong acid (HX); this is together named as nitrous acid.
> Thus, the unstable intermediates are formed from the diazotization of primary aromatic amines, and the counter ion present can be isolated.
> In the last, we can conclude that diazotization leads to the formation of diazonium salts.
> Additional information: The diazonium salts have been used in many applications such as in dye, and pigment industries; as standard reagents in the production of organic compounds; and most important in the field of nanotechnology.
Note: Don’t get confused about sodium nitrite, and a strong acid is named as nitrous acid. It is generated in situ form, and a strong acid can be hydrochloric acid, or sulphuric acid. Now, this can also create a confusion that how the counter ion can be isolated, it will be isolated only if it acts non-nucleophilic.
Complete step by step solution:
> Let us define the diazotization. It is a chemical process involved in organic chemistry.
- It is the conversion of primary aromatic amine into the diazonium salt of amine by the use of nitrous acid; also known as diazotization.
- This reaction was discovered by Peter Griess, and he proposed many reactions having diazonium salts.
Now, we will see the reaction representing the diazotization is

> We can see that this reaction represents the reaction of primary aromatic amines with sodium nitrite, and a strong acid (HX); this is together named as nitrous acid.
> Thus, the unstable intermediates are formed from the diazotization of primary aromatic amines, and the counter ion present can be isolated.
> In the last, we can conclude that diazotization leads to the formation of diazonium salts.
> Additional information: The diazonium salts have been used in many applications such as in dye, and pigment industries; as standard reagents in the production of organic compounds; and most important in the field of nanotechnology.
Note: Don’t get confused about sodium nitrite, and a strong acid is named as nitrous acid. It is generated in situ form, and a strong acid can be hydrochloric acid, or sulphuric acid. Now, this can also create a confusion that how the counter ion can be isolated, it will be isolated only if it acts non-nucleophilic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




