
What is Bond line notation?
Answer
500.4k+ views
Hint: The name bond line notation denotes the bonds in organic molecules represented by lines. The lines are preferably zig-zag lines to show the presence of carbon atoms. It can also be referred to as skeletal structure or skeletal formula.
Complete answer:
Bond line notation is defined as the structural condensed formula of organic compounds. The carbon and hydrogen atoms are not written and do not appear on the structure. Only, the bonds present between the carbon atoms is shown. The end of the structure and the points where the two lines meet i.e. the vertices are where the carbon atoms are present. The number of hydrogens depends on the valency of carbon.
The terminal carbon atoms will have 3 hydrogen atoms whereas the carbon atoms in the chain will have 2 hydrogen atoms.
In this structure, the covalent bonds are represented by a single line for a single bond, double parallel lines for a double bond, and three parallel lines for a triple bond.
The atoms other than carbon and hydrogen can be shown using their symbols.
Let us see some examples of bond line notation:
Now, let’s see the bond line notations of some groups:
Note:
The bond line notations are prepared by eliminating the carbon and hydrogen atoms from the expanded normal representation. They are subsequently small as compared to the structures from which they are derived. The name of the parent chain can be found by counting the number of lines in the bond line notation.
Complete answer:
Bond line notation is defined as the structural condensed formula of organic compounds. The carbon and hydrogen atoms are not written and do not appear on the structure. Only, the bonds present between the carbon atoms is shown. The end of the structure and the points where the two lines meet i.e. the vertices are where the carbon atoms are present. The number of hydrogens depends on the valency of carbon.
The terminal carbon atoms will have 3 hydrogen atoms whereas the carbon atoms in the chain will have 2 hydrogen atoms.
In this structure, the covalent bonds are represented by a single line for a single bond, double parallel lines for a double bond, and three parallel lines for a triple bond.
The atoms other than carbon and hydrogen can be shown using their symbols.
Let us see some examples of bond line notation:
| Name of Organic compound | Normal representation | Bond line notation |
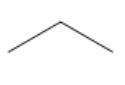
| Propane | 
| 
|
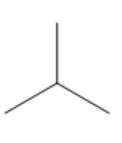
| Isobutane | 
| 
|
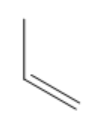
| Propene | 
| 
|
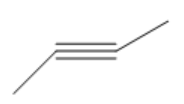
| 2-Butyne | 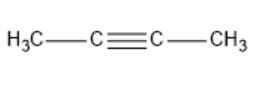
| 
|
Now, let’s see the bond line notations of some groups:
| Name of Organic compound | Normal representation | Bond line notation |
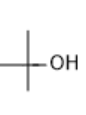
| Tertiary butyl alcohol | 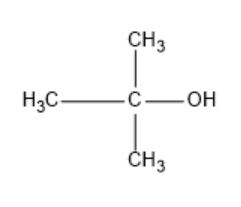
| 
|
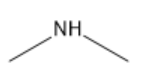
| Dimethylamine | 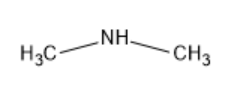
| 
|
Note:
The bond line notations are prepared by eliminating the carbon and hydrogen atoms from the expanded normal representation. They are subsequently small as compared to the structures from which they are derived. The name of the parent chain can be found by counting the number of lines in the bond line notation.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




