
What is achiral carbon atom?
Answer
526.2k+ views
Hint :In the given question firstly we have to define what exactly are the carbon atoms which are achiral and how can we determine it and give the proper definition and the characteristics of it. And now we can also say that they are the carbon atoms which aren’t optically active taking them as the central carbon atom.
Complete Step By Step Answer:
The given question statement asks about the carbon atoms which are achiral in nature and the elements forming them. We have to provide the basic definition and the other important characteristics for these atoms along with the significance in chemistry and isomerism.
The concept of Chirality is important for the purpose of stereochemistry and biochemistry. In this case we can say that Most substances which are relevant to biology are chiral, such as carbohydrates, the amino acids that are the building blocks of proteins, and the nucleic acids. In the case of living organisms we can typically find only one of the two enantiomers of a chiral compound.
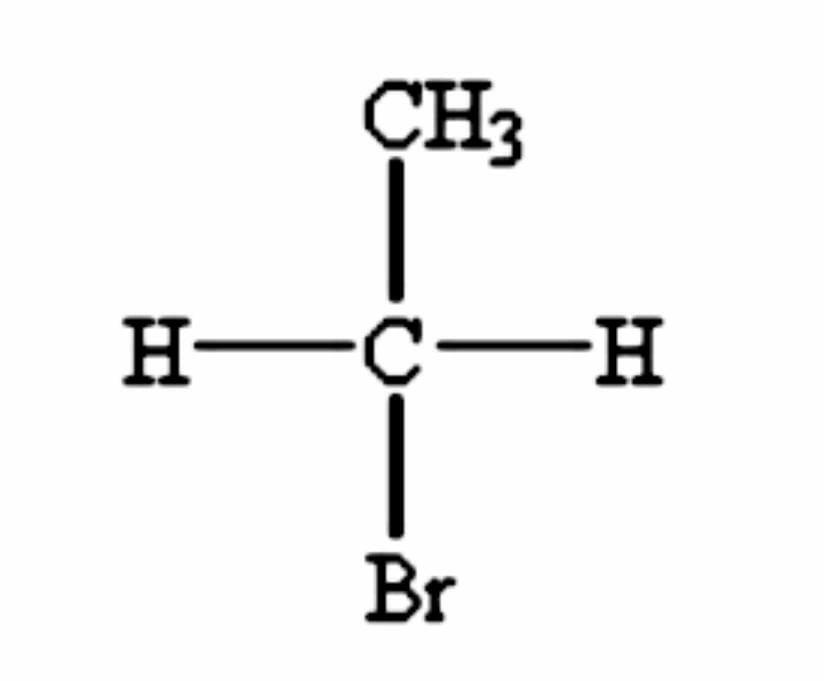
And a molecule is considered chiral if there exists another molecule that is of identical composition of it but which can be arranged in a non-superimposable mirror image to the original one. The presence of an asymmetric carbon atom is often the main and the character defining feature that causes chirality in molecules. There are 2 mirror images of a chiral molecule called enantiomers or optical isomers. Achiral carbon does not have 4 different atoms or groups bonded to the carbon.
Note :
When the optical rotation for an enantiomer is too low for practical measurement, the species is said to exhibit cryptochirality. Chirality is an intrinsic part of the identity of a molecule, so the systematic name includes details of the absolute configuration.
Complete Step By Step Answer:
The given question statement asks about the carbon atoms which are achiral in nature and the elements forming them. We have to provide the basic definition and the other important characteristics for these atoms along with the significance in chemistry and isomerism.
The concept of Chirality is important for the purpose of stereochemistry and biochemistry. In this case we can say that Most substances which are relevant to biology are chiral, such as carbohydrates, the amino acids that are the building blocks of proteins, and the nucleic acids. In the case of living organisms we can typically find only one of the two enantiomers of a chiral compound.

And a molecule is considered chiral if there exists another molecule that is of identical composition of it but which can be arranged in a non-superimposable mirror image to the original one. The presence of an asymmetric carbon atom is often the main and the character defining feature that causes chirality in molecules. There are 2 mirror images of a chiral molecule called enantiomers or optical isomers. Achiral carbon does not have 4 different atoms or groups bonded to the carbon.
Note :
When the optical rotation for an enantiomer is too low for practical measurement, the species is said to exhibit cryptochirality. Chirality is an intrinsic part of the identity of a molecule, so the systematic name includes details of the absolute configuration.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




