
What is a phenoxide ion?
Answer
510.3k+ views
Hint :The conjugate base of phenol is a phenoxide ion. Phenoxide is a conjugate base, meaning it is made up of an acid that has lost its hydrogen. A phenol molecule is what this acid is. The hydroxyl's hydrogen exits and an $O^-$ remains, generating the phenoxide ion oxide ion' component.
Complete Step By Step Answer:
The capacity of phenols to lose hydrogen ions and produce phenoxide ions gives them their acidity. The benzene ring's $sp^2$ hybridised carbon atom connected directly to the hydroxyl group works as an electron-withdrawing group in a phenol molecule. In compared to the hydroxyl group, this $sp^2$ hybridised carbon atom of a benzene ring linked straight to it has a stronger electronegativity. The electron density on the oxygen atom lowers due to the increased electronegativity of this carbon atom compared to the hydroxyl group connected. Increased ionisation of phenols is caused by a reduction in electron density, which enhances the polarity of the O-H bond.
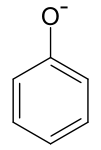
The phenoxide ion is produced as a result. The formation of the phenoxide ion is stabilised by the delocalization of negative charge caused by the benzene ring's resonance. Because charge separation occurs during resonance in the case of phenols, the phenoxide ion is more stable than phenols. In the case of substituted phenols, the acidity increases when the electron-withdrawing group is present. This is owing to the stability of the produced phenoxide ion. If these groups are linked at ortho and para locations, the acidity of phenols rises much more.
Note :
The reason for this is that the phenoxide ion's negative charge is most delocalized in the ortho and para locations of the connected benzene ring.
In the presence of electron-donating groups, however, the acidity of phenols diminishes because the production of the phenoxide ion is prevented.
Complete Step By Step Answer:
The capacity of phenols to lose hydrogen ions and produce phenoxide ions gives them their acidity. The benzene ring's $sp^2$ hybridised carbon atom connected directly to the hydroxyl group works as an electron-withdrawing group in a phenol molecule. In compared to the hydroxyl group, this $sp^2$ hybridised carbon atom of a benzene ring linked straight to it has a stronger electronegativity. The electron density on the oxygen atom lowers due to the increased electronegativity of this carbon atom compared to the hydroxyl group connected. Increased ionisation of phenols is caused by a reduction in electron density, which enhances the polarity of the O-H bond.

The phenoxide ion is produced as a result. The formation of the phenoxide ion is stabilised by the delocalization of negative charge caused by the benzene ring's resonance. Because charge separation occurs during resonance in the case of phenols, the phenoxide ion is more stable than phenols. In the case of substituted phenols, the acidity increases when the electron-withdrawing group is present. This is owing to the stability of the produced phenoxide ion. If these groups are linked at ortho and para locations, the acidity of phenols rises much more.
Note :
The reason for this is that the phenoxide ion's negative charge is most delocalized in the ortho and para locations of the connected benzene ring.
In the presence of electron-donating groups, however, the acidity of phenols diminishes because the production of the phenoxide ion is prevented.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




