
What is a covalent compound?
Answer
519k+ views
Hint: We have to know that the compound that contains covalent bond forms covalent compound. Covalent property of any compound or element or molecule can be studied on the basis of electronegativity. On moving down the group electronegativity decreases and moving along a period the trend gets reversed this will be helpful in answering this question better.
Complete answer:
We have to know that the covalent bond is formed between elements that have electronegativity difference in them. Covalent compound contains non metals thus covalent bond is formed between non-metals since they are electronegative in nature unlike alkali metals or alkaline earth metals which are electropositive. When the electronegativity values are similar between two atoms then covalent compounds are formed. The melting and boiling points of covalent compounds are low since many of the molecules forming covalent compounds are gas or liquid at room temperature. Covalent bonds are easy to break as solid covalent compounds are soft and brittle.
Examples of covalent compounds are: Oxygen, chlorine, methane, water, hydrochloric acid etc.
The bond between two hydrogen atoms forming a hydrogen molecule i.e.\[{H_2}\] is held together by a covalent bond, thus the hydrogen molecule is a covalent compound.
In the case of methane molecules, Carbon is more electronegative than hydrogen atoms thus covalent bond is formed between carbon and hydrogen atoms making methane covalent compound.
In the case of water molecules, Oxygen is more electronegative than hydrogen atom thus hydrogen atoms are electropositive and the bonding between them is covalent bonding due to the electronegativity difference between oxygen and hydrogen atoms.
Note:
The examples shown above are all single covalent bonds. Covalent bonds can be of three types i.e. single covalent bond, double covalent bond and triple covalent bond. Example of single covalent bond is water molecule, example of double covalent bond is carbon dioxide and example of triple covalent bond is nitrogen molecule.
Complete answer:
We have to know that the covalent bond is formed between elements that have electronegativity difference in them. Covalent compound contains non metals thus covalent bond is formed between non-metals since they are electronegative in nature unlike alkali metals or alkaline earth metals which are electropositive. When the electronegativity values are similar between two atoms then covalent compounds are formed. The melting and boiling points of covalent compounds are low since many of the molecules forming covalent compounds are gas or liquid at room temperature. Covalent bonds are easy to break as solid covalent compounds are soft and brittle.
Examples of covalent compounds are: Oxygen, chlorine, methane, water, hydrochloric acid etc.
- 1.

The bond between two hydrogen atoms forming a hydrogen molecule i.e.\[{H_2}\] is held together by a covalent bond, thus the hydrogen molecule is a covalent compound.
2.
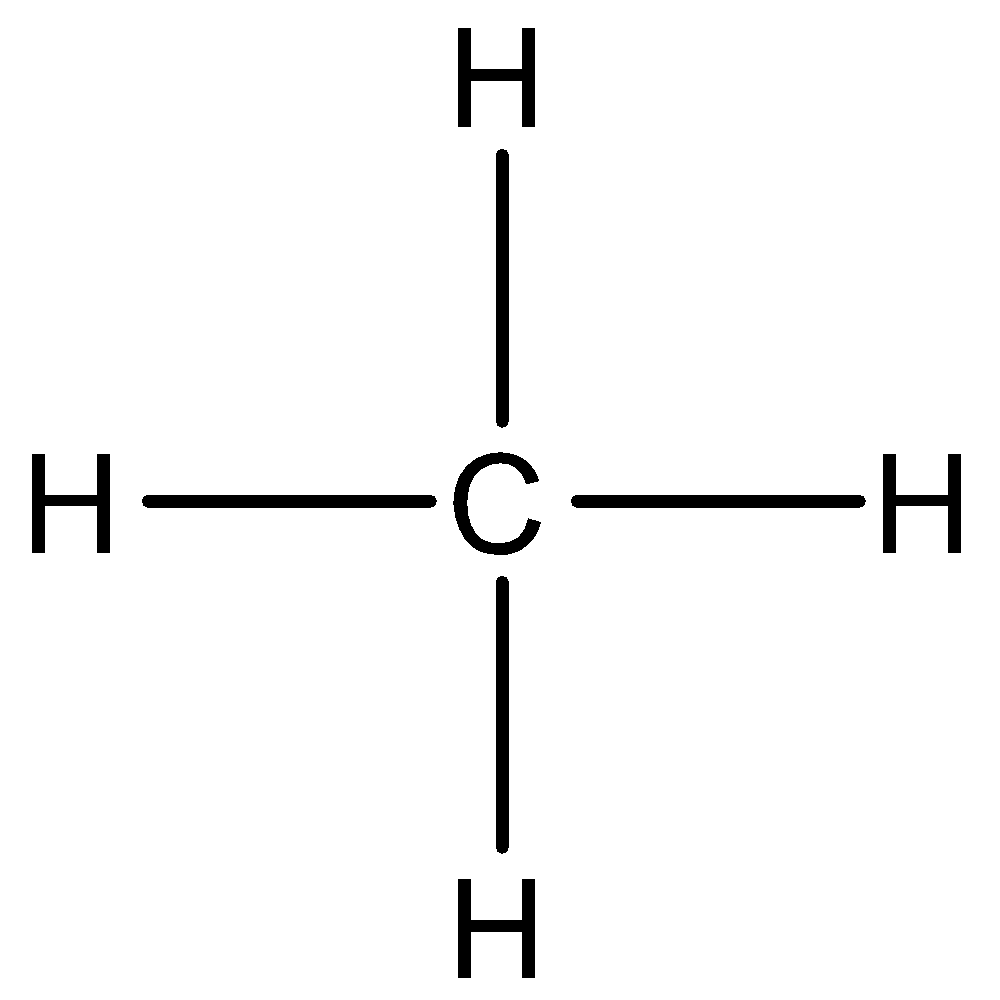

In the case of methane molecules, Carbon is more electronegative than hydrogen atoms thus covalent bond is formed between carbon and hydrogen atoms making methane covalent compound.
3.
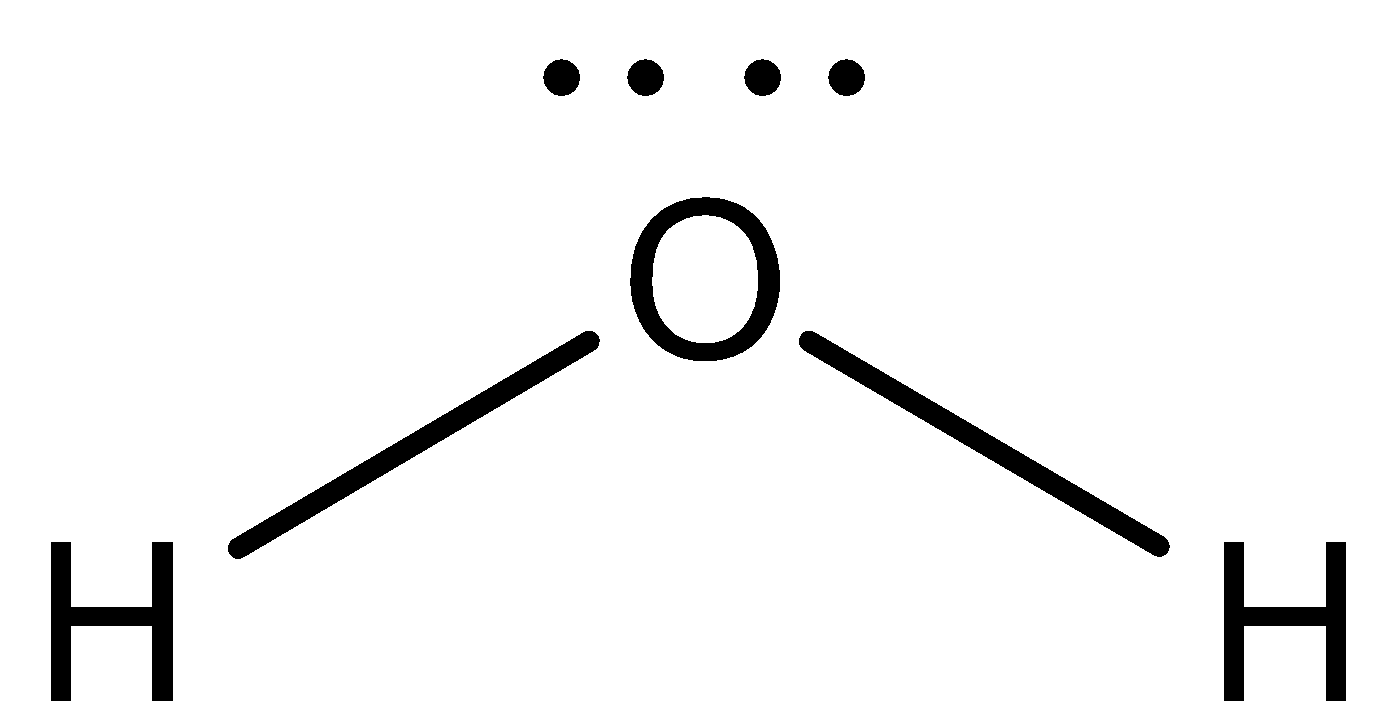

In the case of water molecules, Oxygen is more electronegative than hydrogen atom thus hydrogen atoms are electropositive and the bonding between them is covalent bonding due to the electronegativity difference between oxygen and hydrogen atoms.
Note:
The examples shown above are all single covalent bonds. Covalent bonds can be of three types i.e. single covalent bond, double covalent bond and triple covalent bond. Example of single covalent bond is water molecule, example of double covalent bond is carbon dioxide and example of triple covalent bond is nitrogen molecule.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




