
What is a chromophore?
Answer
519.3k+ views
Hint: The functional groups which impart color to an organic compound are known as the chromophore. Any compound is colored when it is able to absorb light of a certain wavelength and reflects the complementary light which lies in the visible region of the electromagnetic spectrum. It is this reflected light that can be perceived by our eyes.
Complete answer:
When an organic compound absorbs the radiation in the visible part of the electromagnetic spectrum, it appears to be colored. The colored properties associated with the organic compounds are due to the presence of few groups of atoms known as chromophores which absorb visible light photons.
In Greek, chroma means color, and phoron means bearer. A chromophore is usually a group of atoms that are having electron-withdrawing nature, possess unsaturation, and when present in conjugation imparts color to the compound by absorption of visible light. Examples of chromophore include groups such as –
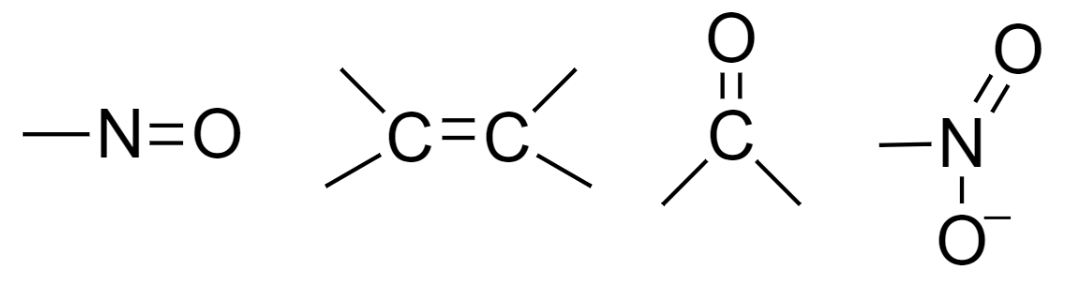
When light falls on a compound, it gets absorbed and results in three types of excitations in the molecule, namely electronic, vibrational and rotational. The compounds undergo electronic excitation in the UV–visible region. In the case of multiple bonded compounds, the π-electrons are responsible for absorption and electronic excitation.
Now, the energy required for the excitation in a molecule is directly related to the frequency of light absorbed. It is given by the following relation –
\[\Delta \text{E}={{\text{E}}_{2}}-{{\text{E}}_{1}}=\text{h }\!\!\nu\!\!\text{ }=\dfrac{\text{hc}}{\text{ }\!\!\lambda\!\!\text{ }}\]
Where \[{{\text{E}}_{1}}\] and \[{{\text{E}}_{2}}\] are energy corresponding to ground state and excited state respectively.
In organic compounds with conjugated multiple bond systems, the delocalization of π-electrons occurs. This delocalization leads to a resonance effect that causes stabilization in the excited state and thus decreases \[\Delta \text{E}\] value. As a result, longer wavelength absorption occurs that belongs to the visible region, and the compound appears colored. The chromophore groups present in a compound cause deepening of color by increasing the number of charged contributing structures during the resonance effect.
Note:
Some groups do not impart color but when present along with chromophore groups are responsible for deepening the color of the compound. These are electron-donating groups and are known as auxochromes. Examples of auxochrome groups are:
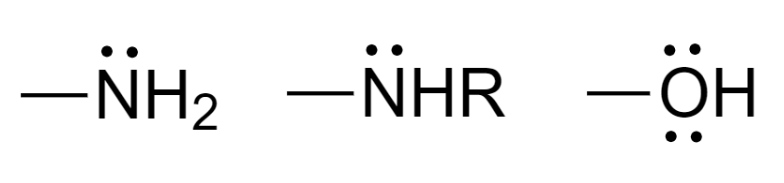
Complete answer:
When an organic compound absorbs the radiation in the visible part of the electromagnetic spectrum, it appears to be colored. The colored properties associated with the organic compounds are due to the presence of few groups of atoms known as chromophores which absorb visible light photons.
In Greek, chroma means color, and phoron means bearer. A chromophore is usually a group of atoms that are having electron-withdrawing nature, possess unsaturation, and when present in conjugation imparts color to the compound by absorption of visible light. Examples of chromophore include groups such as –

When light falls on a compound, it gets absorbed and results in three types of excitations in the molecule, namely electronic, vibrational and rotational. The compounds undergo electronic excitation in the UV–visible region. In the case of multiple bonded compounds, the π-electrons are responsible for absorption and electronic excitation.
Now, the energy required for the excitation in a molecule is directly related to the frequency of light absorbed. It is given by the following relation –
\[\Delta \text{E}={{\text{E}}_{2}}-{{\text{E}}_{1}}=\text{h }\!\!\nu\!\!\text{ }=\dfrac{\text{hc}}{\text{ }\!\!\lambda\!\!\text{ }}\]
Where \[{{\text{E}}_{1}}\] and \[{{\text{E}}_{2}}\] are energy corresponding to ground state and excited state respectively.
In organic compounds with conjugated multiple bond systems, the delocalization of π-electrons occurs. This delocalization leads to a resonance effect that causes stabilization in the excited state and thus decreases \[\Delta \text{E}\] value. As a result, longer wavelength absorption occurs that belongs to the visible region, and the compound appears colored. The chromophore groups present in a compound cause deepening of color by increasing the number of charged contributing structures during the resonance effect.
Note:
Some groups do not impart color but when present along with chromophore groups are responsible for deepening the color of the compound. These are electron-donating groups and are known as auxochromes. Examples of auxochrome groups are:

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




