
What is the valency of silicon atom-
A. 4
B. 3
C. 2
D. 1
Answer
572.4k+ views
Hint: Atom’s chemical properties depend on the atomic number. The valency of an element depends upon the total number of electrons in the outermost shell of an atom. Valence electrons are the electrons that are present in the outermost shell of an atom.
Complete step by step answer:
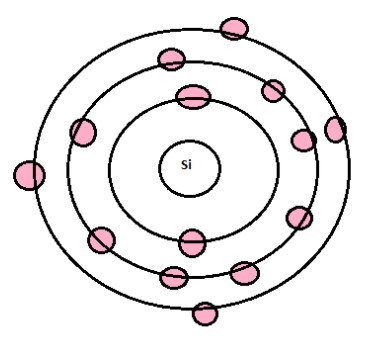
Silicon is a chemical element found in the periodic table which has the symbol Si with an atomic number 14. The atomic structure of silicon makes it an extremely important semiconductor. The atoms in silicon are held in fixed positions by strong forces and only have limited movement.
The valency is determined by the number of valence electrons present in the atom of that element.
Silicon has 4 electrons in the outermost shell. Hence, the valency of silicon atoms is 4.
Thus, the correct answer is option A. i.e., 4.
Additional Information:
Electronic configuration of Silicon is 2, 8, 4. Thus to complete its octet it either needs to lose 4 electrons or gain 4 electrons. Normally it does not do it because this requires a high amount of energy. But it does have the ability to show +4 or -4 valency. To complete the octet, it can share its 4 electrons, hence its valency is 4.
Note:
Silicon is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid and semiconductor. In the periodic table, it is a member of group fourteen. Each silicon atom consists of two oxygen atoms. Carbon is present above the silicon atom and germanium; tin and lead are present below it.
Complete step by step answer:
Silicon is a chemical element found in the periodic table which has the symbol Si with an atomic number 14. The atomic structure of silicon makes it an extremely important semiconductor. The atoms in silicon are held in fixed positions by strong forces and only have limited movement.
The valency is determined by the number of valence electrons present in the atom of that element.
Silicon has 4 electrons in the outermost shell. Hence, the valency of silicon atoms is 4.

Thus, the correct answer is option A. i.e., 4.
Additional Information:
Electronic configuration of Silicon is 2, 8, 4. Thus to complete its octet it either needs to lose 4 electrons or gain 4 electrons. Normally it does not do it because this requires a high amount of energy. But it does have the ability to show +4 or -4 valency. To complete the octet, it can share its 4 electrons, hence its valency is 4.
Note:
Silicon is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid and semiconductor. In the periodic table, it is a member of group fourteen. Each silicon atom consists of two oxygen atoms. Carbon is present above the silicon atom and germanium; tin and lead are present below it.
Recently Updated Pages
Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Computer Science: Engaging Questions & Answers for Success

Class 10 Question and Answer - Your Ultimate Solutions Guide

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Trending doubts
A boat goes 24 km upstream and 28 km downstream in class 10 maths CBSE

State and explain Ohms law class 10 physics CBSE

Distinguish between soap and detergent class 10 chemistry CBSE

a Why did Mendel choose pea plants for his experiments class 10 biology CBSE

What is a "free hit" awarded for in limited-overs cricket?

Draw the diagram of the sectional view of the human class 10 biology CBSE




