
How many valence electrons are used to make sigma bonds in the molecule \[{C_3}{H_6}\]?
Answer
561k+ views
Hint In order to find the number of valence electrons required to make a sigma bond in a molecule \[{C_3}{H_6}\], we must first know what a valence electron is. Valence electrons are those electrons which are present in the outermost orbital or shell.
Complete step by step solution:
Let us first understand what a valence electron is. Valence electrons are those electrons which are present in the outermost orbitals or shells. Those electrons which are present in the inner shells are called the core electrons.
Let us move to the question. There will be formation of two compounds from the molecular formula \[{C_3}{H_6}\]. The two compounds which are formed will be cyclopropane and propene.in the molecular formula \[{C_3}{H_6}\], two chemical elements will be present they are carbon and hydrogen. Carbon is having an atomic number of 6 and it will be having 4 valence electrons in it. As there are three carbon atoms in \[{C_3}{H_6}\], then the total valence electrons present in carbon in \[{C_3}{H_6}\] is 12. Hydrogen is having an atomic number of 1 and it will be having 1 valence electron. As there are six hydrogen atoms in \[{C_3}{H_6}\], then the total valence electrons present in hydrogen in \[{C_3}{H_6}\] is 6. Hence the total valence electron in \[{C_3}{H_6}\] is 18.
The structure for cyclopropane is given below:
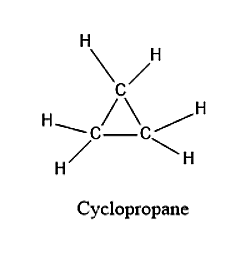
From the structure, we can see that there are 9 bonds present which are formed by mutual sharing of electrons. Hence there are 9 sigma bonds present.
The structure for Propene is given below:
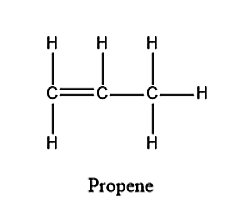
From the structure, we can see that there is a double bond in propene in which one is sigma bond and one is pi bond. Therefore, in propene there are 8 sigma bonds present.
Note: We have to remember certain points such as
- The valence electrons in transition metals exist in the inner shells.
- The valence electrons that are completely filled are chemically inert.
- The valence electrons can absorb or emit energy in the form of photons
Complete step by step solution:
Let us first understand what a valence electron is. Valence electrons are those electrons which are present in the outermost orbitals or shells. Those electrons which are present in the inner shells are called the core electrons.
Let us move to the question. There will be formation of two compounds from the molecular formula \[{C_3}{H_6}\]. The two compounds which are formed will be cyclopropane and propene.in the molecular formula \[{C_3}{H_6}\], two chemical elements will be present they are carbon and hydrogen. Carbon is having an atomic number of 6 and it will be having 4 valence electrons in it. As there are three carbon atoms in \[{C_3}{H_6}\], then the total valence electrons present in carbon in \[{C_3}{H_6}\] is 12. Hydrogen is having an atomic number of 1 and it will be having 1 valence electron. As there are six hydrogen atoms in \[{C_3}{H_6}\], then the total valence electrons present in hydrogen in \[{C_3}{H_6}\] is 6. Hence the total valence electron in \[{C_3}{H_6}\] is 18.
The structure for cyclopropane is given below:

From the structure, we can see that there are 9 bonds present which are formed by mutual sharing of electrons. Hence there are 9 sigma bonds present.
The structure for Propene is given below:

From the structure, we can see that there is a double bond in propene in which one is sigma bond and one is pi bond. Therefore, in propene there are 8 sigma bonds present.
Note: We have to remember certain points such as
- The valence electrons in transition metals exist in the inner shells.
- The valence electrons that are completely filled are chemically inert.
- The valence electrons can absorb or emit energy in the form of photons
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




