
Using the MO diagram of NO, calculate the bond order. Compare it to \[N{O^ + }\]
Answer
527.1k+ views
Hint: A molecular orbital is a mathematical feature in chemistry that describes the position and wavelike activity of an electron in a molecule. This function can be used to quantify chemical and physical properties including the likelihood of finding an electron in a given field.
Complete answer:
The bond order of a compound is an approximation of the total number of chemical bonds present. The discrepancy between bonding and nonbonding electrons in the molecular orbitals can be used to quantify bond order. The bond order of O=O, for example, is 2.
MO of NO:
The atomic number of N is 7 and the atomic number of O is 8. The total number of electrons will be 15.
N has an atomic number of 7 and O has an atomic number of 8. There will be a total of 15 electrons.
The electronic configuration of NO = \[\sigma \left( {1{s^2}} \right){\sigma ^*}\left( {1{s^2}} \right)\sigma \left( {2{s^2}} \right){\sigma ^*}\left( {2{s^2}} \right)\sigma {\left( {2{p_x}} \right)^2}\pi {\left( {2{p_y}} \right)^2}\pi {\left( {2{p_z}} \right)^2}{\pi ^*}{\left( {2{p_y}} \right)^1}\]
It can be shown that one electron is unpaired. As a result, it's a paramagnetic substance. NO's orbital diagram is as follows.
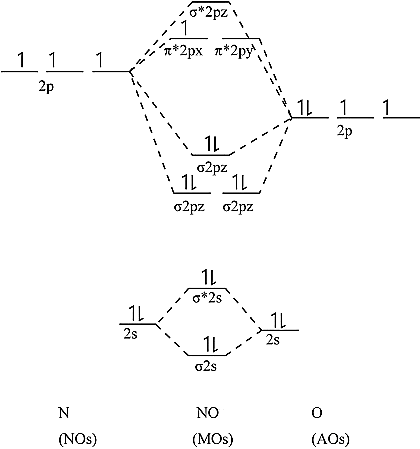
Bond order calculation:
The number of bonds between two atoms is referred to as bond order. Linus Pauling was the one who first proposed it. The higher a bond's bond order, the more stable the bond. As a result, a single bond is weaker than a double bond, which in turn is weaker than a triple bond. We'll need details about the molecule's molecular orbital diagram to figure out the bond sequence. The electrons are present in bonding orbitals and which are present in antibonding orbitals are seen in a molecular orbital diagram. Bond order can also be expressed as a dfractional number in some situations.
\[{\text{ Bond order }} = \dfrac{{{\text{ Bonding electron }} - {\text{ non bonding electron }}}}{2}\]
\[{\text{ Bond order }} = \dfrac{{10 - 5}}{2}\]
\[{\text{ Bond order }} = \dfrac{5}{2}\]
Bond order = 2.5
Hence, the bond order of NO will be 2.5
Bond order \[N{O^ + }\]
N has an atomic number of 7 and O has an atomic number of 8. In helium, there are five valence electrons, and in oxygen, there are six. The overall number of electrons will be diminished by one due to the presence of +1 charge, to 14, with 10 bonding electrons and 4 non-bonding electrons.
The electronic configuration of NO⁺ =
\[\sigma \left( {1{s^2}} \right){\sigma ^*}\left( {1{s^2}} \right)\sigma \left( {2{s^2}} \right){\sigma ^*}\left( {2{s^2}} \right)\sigma {\left( {2{p_x}} \right)^2}\pi {\left( {2{p_y}} \right)^2}\pi {\left( {2{p_z}} \right)^2}\]
Many of the electrons are paired, as can be shown. As a result, it's a diamagnetic substance.
Bond order calculation.
\[{\text{ Bond order }} = \dfrac{{{\text{ Number of bonding electron }} - {\text{ Number of antibonding electron }}}}{2}\]
\[{\text{ Bond order }} = \dfrac{{10 - 4}}{2}\]
\[{\text{ Bond order }} = \dfrac{6}{2}\]
Bond order = 3
Hence, the bond order of \[N{O^ + }\] will be 3
Note:
Electrons in a molecule are not attributed to particular chemical bonds between atoms in molecular orbital theory, but are often treated as passing under the direction of the atomic nuclei in the whole molecule. The spatial and energetic properties of electrons are defined by quantum mechanics as molecular orbitals, which surround two or more atoms in a molecule and contain valence electrons between them.
Complete answer:
The bond order of a compound is an approximation of the total number of chemical bonds present. The discrepancy between bonding and nonbonding electrons in the molecular orbitals can be used to quantify bond order. The bond order of O=O, for example, is 2.
MO of NO:
The atomic number of N is 7 and the atomic number of O is 8. The total number of electrons will be 15.
N has an atomic number of 7 and O has an atomic number of 8. There will be a total of 15 electrons.
The electronic configuration of NO = \[\sigma \left( {1{s^2}} \right){\sigma ^*}\left( {1{s^2}} \right)\sigma \left( {2{s^2}} \right){\sigma ^*}\left( {2{s^2}} \right)\sigma {\left( {2{p_x}} \right)^2}\pi {\left( {2{p_y}} \right)^2}\pi {\left( {2{p_z}} \right)^2}{\pi ^*}{\left( {2{p_y}} \right)^1}\]
It can be shown that one electron is unpaired. As a result, it's a paramagnetic substance. NO's orbital diagram is as follows.

Bond order calculation:
The number of bonds between two atoms is referred to as bond order. Linus Pauling was the one who first proposed it. The higher a bond's bond order, the more stable the bond. As a result, a single bond is weaker than a double bond, which in turn is weaker than a triple bond. We'll need details about the molecule's molecular orbital diagram to figure out the bond sequence. The electrons are present in bonding orbitals and which are present in antibonding orbitals are seen in a molecular orbital diagram. Bond order can also be expressed as a dfractional number in some situations.
\[{\text{ Bond order }} = \dfrac{{{\text{ Bonding electron }} - {\text{ non bonding electron }}}}{2}\]
\[{\text{ Bond order }} = \dfrac{{10 - 5}}{2}\]
\[{\text{ Bond order }} = \dfrac{5}{2}\]
Bond order = 2.5
Hence, the bond order of NO will be 2.5
Bond order \[N{O^ + }\]
N has an atomic number of 7 and O has an atomic number of 8. In helium, there are five valence electrons, and in oxygen, there are six. The overall number of electrons will be diminished by one due to the presence of +1 charge, to 14, with 10 bonding electrons and 4 non-bonding electrons.
The electronic configuration of NO⁺ =
\[\sigma \left( {1{s^2}} \right){\sigma ^*}\left( {1{s^2}} \right)\sigma \left( {2{s^2}} \right){\sigma ^*}\left( {2{s^2}} \right)\sigma {\left( {2{p_x}} \right)^2}\pi {\left( {2{p_y}} \right)^2}\pi {\left( {2{p_z}} \right)^2}\]
Many of the electrons are paired, as can be shown. As a result, it's a diamagnetic substance.
Bond order calculation.
\[{\text{ Bond order }} = \dfrac{{{\text{ Number of bonding electron }} - {\text{ Number of antibonding electron }}}}{2}\]
\[{\text{ Bond order }} = \dfrac{{10 - 4}}{2}\]
\[{\text{ Bond order }} = \dfrac{6}{2}\]
Bond order = 3
Hence, the bond order of \[N{O^ + }\] will be 3
Note:
Electrons in a molecule are not attributed to particular chemical bonds between atoms in molecular orbital theory, but are often treated as passing under the direction of the atomic nuclei in the whole molecule. The spatial and energetic properties of electrons are defined by quantum mechanics as molecular orbitals, which surround two or more atoms in a molecule and contain valence electrons between them.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

The largest wind power cluster is located in the state class 11 social science CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

Which among the following are examples of coming together class 11 social science CBSE




