
Using Huckel’s rule, prove that Naphthalene is aromatic.
Answer
596.1k+ views
Hint: According to the Huckel’s rule of aromaticity, if a cyclic conjugated compound is planar and has (4n+2) π electrons, then the compound is said to be an aromatic compound. In an aromatic compound, all the π electrons are delocalized and this phenomenon is known as resonance. An aromatic compound is always more stable than an aliphatic counterpart.
Complete step by step answer:
In order to determine whether a compound is aromatic or not in terms of Huckel’s rule, let us look at the postulates of Huckel’s rule of aromaticity.
According to the Huckel’s rule of aromaticity, the basic criteria for a compound to be aromatic are:
(1) The molecule must be a cyclic molecule (the molecule must have a ring system).
(2) The molecule must possess (4n+2) number of π electrons (where n is zero or any positive integer number).
(3) The molecule must have a fully conjugated system (all the atoms in the ring must be $s{p^2}$ hybridized). All the π electrons must be fully delocalized in the ring system.
(4) The molecule must be planner at all costs (all the atoms in the ring system must be in the same plane).
If a cyclic compound follows the above criteria, then the molecule is said to be an aromatic molecule.
Now, naphthalene is the simplest bicyclic aromatic compound having the molecular formula ${C_{10}}{H_8}$. A naphthalene molecule consists of two benzene rings and they are fused together. The basic structure of a naphthalene molecule is given below:
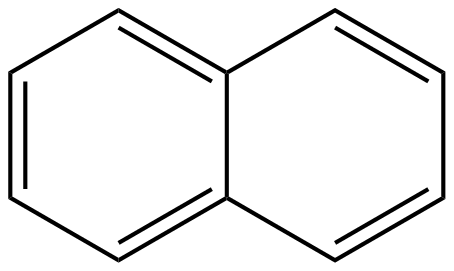
Now, let’s judge where all the postulates of Huckel’s rule apply to naphthalene or not.
-First of all, a naphthalene molecule is a cyclic molecule which means it has a ring system (a naphthalene molecule has two benzene rings fused together). Hence, it is following the first criteria.
-The molecule is having a total of 10 π electrons in the ring system. Hence, it is following the second criteria (4n+2 electrons, where n=2).
-All the π electrons are fully delocalized in the ring system of a naphthalene molecule. It is having a fully conjugated system with all the carbon atoms $s{p^2}$ hybridized. Hence, it is also following the third criteria.
-The naphthalene molecule is fully planner which means all the atoms are in the same plane. Thus, it is following the fourth criteria as well.
Thus, a naphthalene molecule is following all the essential criteria of Huckel’s rule. Hence, according to Huckel's rule of aromaticity, naphthalene is an aromatic compound.
Note: While applying the Huckel’s rule, the student has to remember that the value of n (in 4n+2 electrons) must be a positive integer. In order for a compound to be aromatic, all the criteria must be fulfilled. There are many cyclic compounds which have the required (4n+2) π electrons, but they are not fully conjugated. Many students consider them as aromatic compounds which is wrong.
Complete step by step answer:
In order to determine whether a compound is aromatic or not in terms of Huckel’s rule, let us look at the postulates of Huckel’s rule of aromaticity.
According to the Huckel’s rule of aromaticity, the basic criteria for a compound to be aromatic are:
(1) The molecule must be a cyclic molecule (the molecule must have a ring system).
(2) The molecule must possess (4n+2) number of π electrons (where n is zero or any positive integer number).
(3) The molecule must have a fully conjugated system (all the atoms in the ring must be $s{p^2}$ hybridized). All the π electrons must be fully delocalized in the ring system.
(4) The molecule must be planner at all costs (all the atoms in the ring system must be in the same plane).
If a cyclic compound follows the above criteria, then the molecule is said to be an aromatic molecule.
Now, naphthalene is the simplest bicyclic aromatic compound having the molecular formula ${C_{10}}{H_8}$. A naphthalene molecule consists of two benzene rings and they are fused together. The basic structure of a naphthalene molecule is given below:

Now, let’s judge where all the postulates of Huckel’s rule apply to naphthalene or not.
-First of all, a naphthalene molecule is a cyclic molecule which means it has a ring system (a naphthalene molecule has two benzene rings fused together). Hence, it is following the first criteria.
-The molecule is having a total of 10 π electrons in the ring system. Hence, it is following the second criteria (4n+2 electrons, where n=2).
-All the π electrons are fully delocalized in the ring system of a naphthalene molecule. It is having a fully conjugated system with all the carbon atoms $s{p^2}$ hybridized. Hence, it is also following the third criteria.
-The naphthalene molecule is fully planner which means all the atoms are in the same plane. Thus, it is following the fourth criteria as well.
Thus, a naphthalene molecule is following all the essential criteria of Huckel’s rule. Hence, according to Huckel's rule of aromaticity, naphthalene is an aromatic compound.
Note: While applying the Huckel’s rule, the student has to remember that the value of n (in 4n+2 electrons) must be a positive integer. In order for a compound to be aromatic, all the criteria must be fulfilled. There are many cyclic compounds which have the required (4n+2) π electrons, but they are not fully conjugated. Many students consider them as aromatic compounds which is wrong.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

The largest wind power cluster is located in the state class 11 social science CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

Which among the following are examples of coming together class 11 social science CBSE




