
How do you use the Lewis dot structure to represent hydrogen carbonate?
Answer
544.5k+ views
Hint: So the lewis dot structure is defined as the graphical representation of the electrons which have been distributed around the atom. The Lewis dot structure is important to learn because it helps in predicting the number of bonds and the types of bonds which have to be formed around the atom. It also helps in predicting the geometry of the Lewis dot structure.
Complete step-by-step answer:
So to make the lewis dot structure we should first calculate the valence electrons. So the hydrogen carbonate \[HC{O_3}^ - \]has 1 valence electron of hydrogen, 4 valence electrons of carbon and each oxygen has 6 valence electrons and the atoms involved is 3 so 18 valence electrons from oxygen and the presence of negative charge so 1 more. So in total we have 24 valence electrons.
Now let us make the Lewis dot structure of the hydrogen carbonate
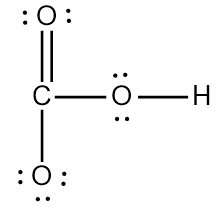
Here we see that six electrons have been used in forming the single bond and two more electrons have been used in forming the double bond. So in total eight valence electrons have been used and the rest 14 electrons or the 7 lone pairs have not been used.
Note: We can calculate the formal charge with the help of the lewis dot structure. The formal charge is the theoretical charge which does not indicate the real charge separation present in the molecule. It also helps in selection of the lowest energy structure. The lowest energy structure helps in predicting the major product of the reaction.
Complete step-by-step answer:
So to make the lewis dot structure we should first calculate the valence electrons. So the hydrogen carbonate \[HC{O_3}^ - \]has 1 valence electron of hydrogen, 4 valence electrons of carbon and each oxygen has 6 valence electrons and the atoms involved is 3 so 18 valence electrons from oxygen and the presence of negative charge so 1 more. So in total we have 24 valence electrons.
Now let us make the Lewis dot structure of the hydrogen carbonate

Here we see that six electrons have been used in forming the single bond and two more electrons have been used in forming the double bond. So in total eight valence electrons have been used and the rest 14 electrons or the 7 lone pairs have not been used.
Note: We can calculate the formal charge with the help of the lewis dot structure. The formal charge is the theoretical charge which does not indicate the real charge separation present in the molecule. It also helps in selection of the lowest energy structure. The lowest energy structure helps in predicting the major product of the reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




