
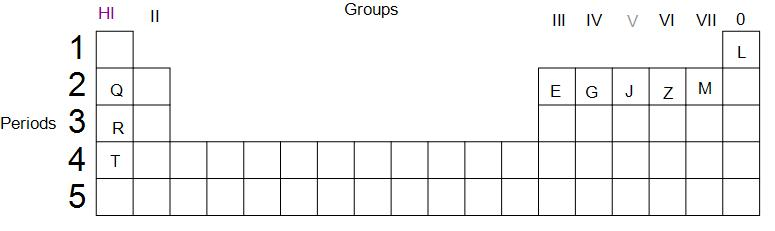
Use the letters only written in the periodic table given below to answer the questions that follow:
(i) State the number of valence electrons in atom J
(ii) Which element shown forms ions with a single negative charge?
(iii) Which metallic element is more reactive than R.
(iv) Which element has its electrons arranged in four shells?

Answer
583.8k+ views
Hint:To answer this question, you must recall the periodic properties and the arrangement of elements in the periodic tables. The group number of an element signifies the number of valence electrons in its atom. The period number of an element gives us the number of shells in the atom.
Complete step by step answer:
(i) We are supposed to find the number of valence electrons in the atom J.
J is a p block element. Before electrons start to fill in its p orbital, two electrons are filled in the s orbital. Since we can see that J is the third p block element in the second period, we can infer that, there are 3 electrons in the p orbital of its valence shell and 2 electrons in the s-orbital of the valence shell. Thus, we can say that the group of an element is the same as the number of electrons present in its valence shell. Therefore, the number of valence electrons in J is five.
(ii) M is an element of group 7. Thus, it has seven valence electrons. To attain stability and noble gas like configuration, it needs to gain one electron. Thus, M forms ions with a single negative charge.
(iii) As we go down the group, the metallic property increases as the size of an atom increases and losing an electron becomes relatively easier. Thus, T is a more reactive metal than R.
(iv) Since we know that the period number of an element gives us the number of shells in the atom, thus T is the element that has its electrons arranged in four valence shells.
Note:
The periodic table can be used to derive relationships between various physical and chemical properties and behaviours of elements. The modern periodic table provides an essential framework that helps in the analysis of various chemical reactions, and is used widely in the fields of chemistry, nuclear physics and other sciences.
Complete step by step answer:
(i) We are supposed to find the number of valence electrons in the atom J.
J is a p block element. Before electrons start to fill in its p orbital, two electrons are filled in the s orbital. Since we can see that J is the third p block element in the second period, we can infer that, there are 3 electrons in the p orbital of its valence shell and 2 electrons in the s-orbital of the valence shell. Thus, we can say that the group of an element is the same as the number of electrons present in its valence shell. Therefore, the number of valence electrons in J is five.
(ii) M is an element of group 7. Thus, it has seven valence electrons. To attain stability and noble gas like configuration, it needs to gain one electron. Thus, M forms ions with a single negative charge.
(iii) As we go down the group, the metallic property increases as the size of an atom increases and losing an electron becomes relatively easier. Thus, T is a more reactive metal than R.
(iv) Since we know that the period number of an element gives us the number of shells in the atom, thus T is the element that has its electrons arranged in four valence shells.
Note:
The periodic table can be used to derive relationships between various physical and chemical properties and behaviours of elements. The modern periodic table provides an essential framework that helps in the analysis of various chemical reactions, and is used widely in the fields of chemistry, nuclear physics and other sciences.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




