
Urotropine is formed by the action of ammonia on which of the following?
${\text{A}}{\text{.}}$ Acetaldehyde
${\text{B}}{\text{.}}$ Formaldehyde
${\text{C}}{\text{.}}$ Acetone
${\text{D}}{\text{.}}$ Phenol
Answer
615.6k+ views
Hint- Here we will proceed by writing down some properties of ammonia along with a figure showing its structure. Then, we will be taking the chemical compounds given in the options and react them with ammonia and will see the product formed. We will do this until urotropine is formed.
Complete answer:
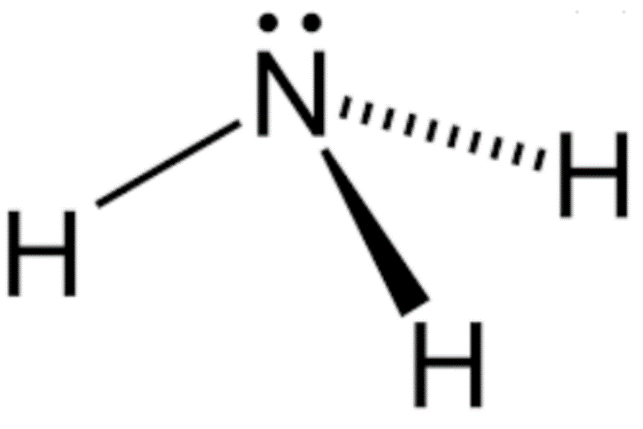
Ammonia is an alkaline colorless gas that is one of the most common chemical compounds containing nitrogen. It is an irritant with a distinctive pungent odor which is commonly used in industry. The ammonia structure is shown in figure.
Often used in the manufacture of industrial explosives such as trinitrotoluene (TNT), nitroglycerin, and nitrocellulose, ammonia.
${\text{A}}{\text{.}}$ When ammonia (${\text{N}}{{\text{H}}_3}$) reacts with acetaldehyde (${\text{C}}{{\text{H}}_3}{\text{CHO}}$), acetaldehyde ammonia trimer [${\left( {{\text{C}}{{\text{H}}_3}{\text{CHNH}}} \right)_3}$] is formed along with water molecules (\[{{\text{H}}_2}{\text{O}}\]). Acetaldehyde ammonia trimer is a trimeric species and is colorless but due to oxidation degradation samples frequently look light yellow or slightly beige. The respective balanced reaction is shown below.
\[{\text{3C}}{{\text{H}}_3}{\text{CHO}} + 3{\text{N}}{{\text{H}}_3} \to {\left( {{\text{C}}{{\text{H}}_3}{\text{CHNH}}} \right)_3} + 3{{\text{H}}_2}{\text{O}}\]
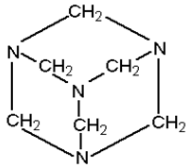
${\text{B}}{\text{.}}$ When ammonia (${\text{N}}{{\text{H}}_3}$) reacts with formaldehyde (${\text{HCHO}}$),hexamethylenetetramine [${\left( {{\text{C}}{{\text{H}}_2}} \right)_6}{{\text{N}}_4}$] is formed along with water molecules (\[{{\text{H}}_2}{\text{O}}\]). A heterocyclic organic compound is hexamethylenetetramine, or methenamine, also known as hexamine or urotropine. Urotropine is a water soluble compound with a structure like a circle, as shown in the figure. This is widely used for treating infection in the urinary tract. The balanced reaction involved is shown below.
${\text{6HCHO}} + {\text{4N}}{{\text{H}}_3} \to {\left( {{\text{C}}{{\text{H}}_2}} \right)_6}{{\text{N}}_4} + 6{{\text{H}}_2}{\text{O}}$
Therefore, we can clearly see that urotropine is formed by the action of ammonia on formaldehyde.
Hence, option B is correct.
Note- Urotropine has an anti-infective activity that is derived from the gradual release by hydrolysis of 0.2 molars of formaldehyde at acid pH. It is used to treat concomitant odour and unnecessary sweating in the form of spray and cream. This is also used as a food preservative, which helps avoid vulcanized rubber as well.


Complete answer:
Ammonia is an alkaline colorless gas that is one of the most common chemical compounds containing nitrogen. It is an irritant with a distinctive pungent odor which is commonly used in industry. The ammonia structure is shown in figure.
Often used in the manufacture of industrial explosives such as trinitrotoluene (TNT), nitroglycerin, and nitrocellulose, ammonia.
${\text{A}}{\text{.}}$ When ammonia (${\text{N}}{{\text{H}}_3}$) reacts with acetaldehyde (${\text{C}}{{\text{H}}_3}{\text{CHO}}$), acetaldehyde ammonia trimer [${\left( {{\text{C}}{{\text{H}}_3}{\text{CHNH}}} \right)_3}$] is formed along with water molecules (\[{{\text{H}}_2}{\text{O}}\]). Acetaldehyde ammonia trimer is a trimeric species and is colorless but due to oxidation degradation samples frequently look light yellow or slightly beige. The respective balanced reaction is shown below.
\[{\text{3C}}{{\text{H}}_3}{\text{CHO}} + 3{\text{N}}{{\text{H}}_3} \to {\left( {{\text{C}}{{\text{H}}_3}{\text{CHNH}}} \right)_3} + 3{{\text{H}}_2}{\text{O}}\]
${\text{B}}{\text{.}}$ When ammonia (${\text{N}}{{\text{H}}_3}$) reacts with formaldehyde (${\text{HCHO}}$),hexamethylenetetramine [${\left( {{\text{C}}{{\text{H}}_2}} \right)_6}{{\text{N}}_4}$] is formed along with water molecules (\[{{\text{H}}_2}{\text{O}}\]). A heterocyclic organic compound is hexamethylenetetramine, or methenamine, also known as hexamine or urotropine. Urotropine is a water soluble compound with a structure like a circle, as shown in the figure. This is widely used for treating infection in the urinary tract. The balanced reaction involved is shown below.
${\text{6HCHO}} + {\text{4N}}{{\text{H}}_3} \to {\left( {{\text{C}}{{\text{H}}_2}} \right)_6}{{\text{N}}_4} + 6{{\text{H}}_2}{\text{O}}$
Therefore, we can clearly see that urotropine is formed by the action of ammonia on formaldehyde.
Hence, option B is correct.
Note- Urotropine has an anti-infective activity that is derived from the gradual release by hydrolysis of 0.2 molars of formaldehyde at acid pH. It is used to treat concomitant odour and unnecessary sweating in the form of spray and cream. This is also used as a food preservative, which helps avoid vulcanized rubber as well.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




