
How many unpaired electrons are in an iron atom?
Answer
548.1k+ views
Hint: The nucleus of the atom contains the protons and the neutrons. The outermost regions of the atom are called electron shells and contain the electrons which are negatively charged. Electrons revolve in different orbits having different energy levels. For arrangement of electrons in each shell, Bohr bury rule is applied.
Complete answer:
- Iron has the atomic number 26 and the electronic configuration is shown below
\begin{align*}1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}4s^{2}3d^{6}\end{align*}
- We will fill up the corresponding orbitals with electrons until you find out the electron arrangement, any left with one is called unpaired electron. The box figure of the outer electronic configuration would appear as
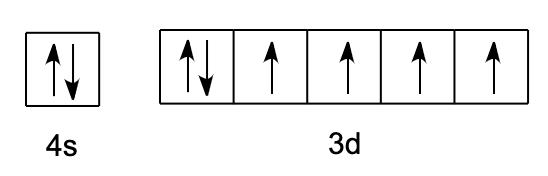
- Therefore, the number of unpaired electrons in iron is 4.
Additional information:
Each atomic orbital of an electron has capacity to contain two electrons which are of opposite spin value i.e., if one electron is in the state of clockwise then another will be in anticlockwise state and this was explained by Pauli exclusion principle.
Note:
Whenever two electrons are paired together in an orbital, or their total spin is zero, they are diamagnetic electrons. Atoms with all diamagnetic electrons are called diamagnetic atoms. A paramagnetic electron is an unpaired electron. An atom is considered paramagnetic if even one orbital has a net spin. Always make sure to remember the concepts of pairing and that is going to help you in understanding the concept of diamagnetic and paramagnetic nature of a substance that will be further useful.
Complete answer:
- Iron has the atomic number 26 and the electronic configuration is shown below
\begin{align*}1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}4s^{2}3d^{6}\end{align*}
- We will fill up the corresponding orbitals with electrons until you find out the electron arrangement, any left with one is called unpaired electron. The box figure of the outer electronic configuration would appear as

- Therefore, the number of unpaired electrons in iron is 4.
Additional information:
Each atomic orbital of an electron has capacity to contain two electrons which are of opposite spin value i.e., if one electron is in the state of clockwise then another will be in anticlockwise state and this was explained by Pauli exclusion principle.
Note:
Whenever two electrons are paired together in an orbital, or their total spin is zero, they are diamagnetic electrons. Atoms with all diamagnetic electrons are called diamagnetic atoms. A paramagnetic electron is an unpaired electron. An atom is considered paramagnetic if even one orbital has a net spin. Always make sure to remember the concepts of pairing and that is going to help you in understanding the concept of diamagnetic and paramagnetic nature of a substance that will be further useful.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




