
How many types of carbon atoms are present in \[2,2,3\]-trimethylpentane?
A.One
B.Two
C.Three
D.Four
Answer
478.2k+ views
Hint: The carbon atoms can be classified as primary, secondary, tertiary, quaternary, etc. depending upon the number of carbon atoms it is bonded to. The type of carbon atom present can be found out using the structure of the compounds.
Complete answer:
The question is to count the types of carbon atoms present in the given compound. The given compound is \[2,2,3\]-trimethylpentane. For finding the types of carbon atoms first let us take a look at the structure of \[2,2,3\]-trimethylpentane. Its structure is as follows:
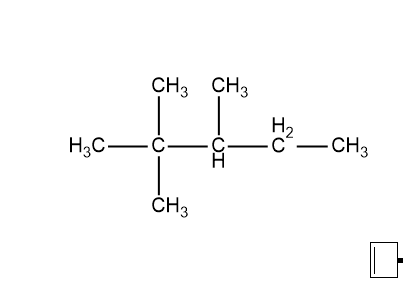
It is known that the different types of carbon are primary, secondary, tertiary, and quaternary. So let us see whether all of these are present in the above-given compound or not.
The first carbon is attached to the other carbon atom through only one bond so this will be the primary carbon. The second carbon is attached to four other carbon atoms thus this carbon will be quaternary. Next, the third carbon is attached to three other carbons so this will be tertiary. The fourth carbon is attached to two other carbon atoms thus this carbon will be a secondary carbon. And the final carbon is again a primary carbon as it is attached to only one other carbon atom.
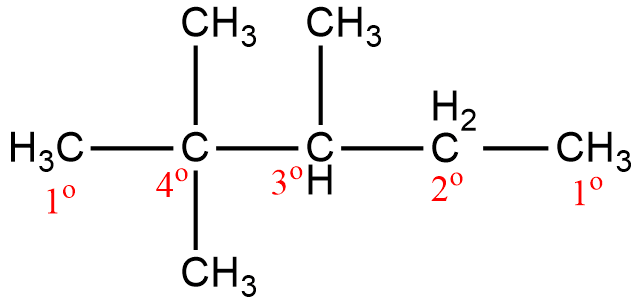
Hence, we can say that the compound \[2,2,3\]-trimethylpentane has four types of carbon atoms.
Therefore the correct option is D. Four
Note:
The classification of carbon atoms into primary, secondary, tertiary, and quaternary only applies to saturated carbons and not in unsaturated carbons. This classification scheme is important as it also applies in classifying organic compounds with different functional groups.
Complete answer:
The question is to count the types of carbon atoms present in the given compound. The given compound is \[2,2,3\]-trimethylpentane. For finding the types of carbon atoms first let us take a look at the structure of \[2,2,3\]-trimethylpentane. Its structure is as follows:

It is known that the different types of carbon are primary, secondary, tertiary, and quaternary. So let us see whether all of these are present in the above-given compound or not.
The first carbon is attached to the other carbon atom through only one bond so this will be the primary carbon. The second carbon is attached to four other carbon atoms thus this carbon will be quaternary. Next, the third carbon is attached to three other carbons so this will be tertiary. The fourth carbon is attached to two other carbon atoms thus this carbon will be a secondary carbon. And the final carbon is again a primary carbon as it is attached to only one other carbon atom.

Hence, we can say that the compound \[2,2,3\]-trimethylpentane has four types of carbon atoms.
Therefore the correct option is D. Four
Note:
The classification of carbon atoms into primary, secondary, tertiary, and quaternary only applies to saturated carbons and not in unsaturated carbons. This classification scheme is important as it also applies in classifying organic compounds with different functional groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




