
Two systems are in thermal equilibrium. The quantity which is common for them is
A. Heat
B. Momentum
C. specific heat
D. temperature
Answer
594k+ views
Hint: Zeroth law of thermodynamics states that two systems A and B, which are separately in thermal equilibrium with a third system C, are also in thermal equilibrium with each other. Any isolated system is in thermodynamic equilibrium.
Complete step by step answer:
Consider two systems A and B, separated by a wall that does not allow any exchange of energy between them. Such a wall is known as an insulating wall or adiabatic wall. The third system C is separated from system A and B by a conducting or diathermic wall.
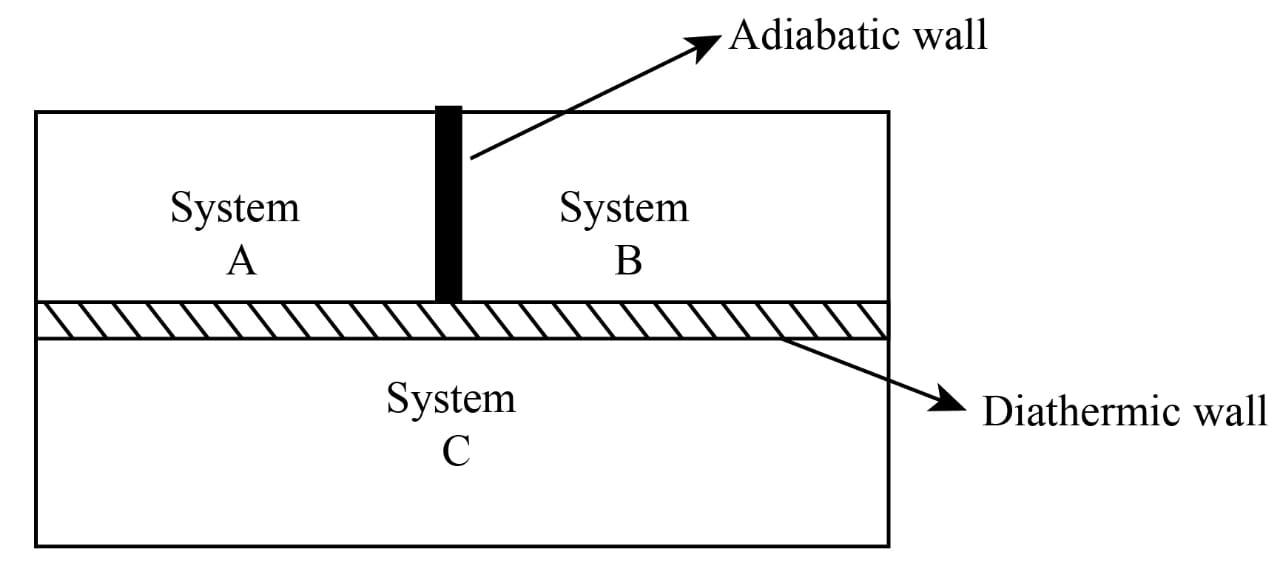
Since energy can be exchanged between the system A and C, so both system A and C are in thermal equilibrium. Similarly, energy can be exchanged between systems B and C. So both B and C are in thermal equilibrium. When the adiabatic wall between the systems A and B are removed, no energy transfer can take place between them because the temperature is the same; this shows that the two systems A and B are in thermal equilibrium; this observation leads to an important law known as Zeroth law of thermodynamics.
Hence, the correct option is (D).
Note:
Concept of temperature from Zeroth law of thermodynamics:
According to law, if system A is in thermal equilibrium with a system C,
Then the temperature of system A = Temperature of system C
Similarly, if system B is in thermal equilibrium with system C,
The temperature of system B = temperature of system C
Now, from the above relation, we conclude that,
The temperature of system A = Temperature of system B
Therefore, the temperature of a system is a physical quantity, equality of which is the only condition for thermal equilibrium.
Complete step by step answer:
Consider two systems A and B, separated by a wall that does not allow any exchange of energy between them. Such a wall is known as an insulating wall or adiabatic wall. The third system C is separated from system A and B by a conducting or diathermic wall.

Since energy can be exchanged between the system A and C, so both system A and C are in thermal equilibrium. Similarly, energy can be exchanged between systems B and C. So both B and C are in thermal equilibrium. When the adiabatic wall between the systems A and B are removed, no energy transfer can take place between them because the temperature is the same; this shows that the two systems A and B are in thermal equilibrium; this observation leads to an important law known as Zeroth law of thermodynamics.
Hence, the correct option is (D).
Note:
Concept of temperature from Zeroth law of thermodynamics:
According to law, if system A is in thermal equilibrium with a system C,
Then the temperature of system A = Temperature of system C
Similarly, if system B is in thermal equilibrium with system C,
The temperature of system B = temperature of system C
Now, from the above relation, we conclude that,
The temperature of system A = Temperature of system B
Therefore, the temperature of a system is a physical quantity, equality of which is the only condition for thermal equilibrium.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




